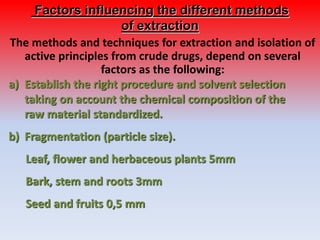
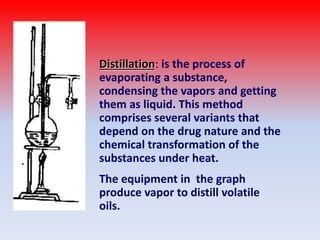
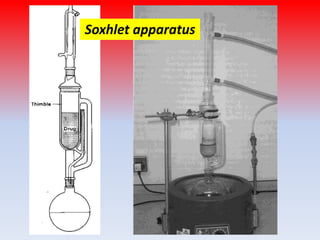
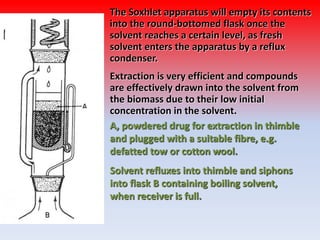
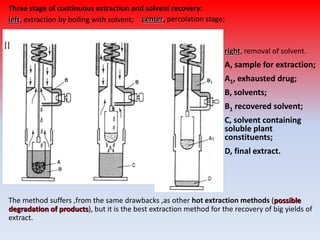
The document discusses various methods for extracting components from medicinal plants. It describes maceration, where fragmented plant material soaks in solvent over 2-14 days. Digestion is similar but involves slight heating. Infusion and decoction extract using hot or boiling water. Distillation extracts volatile oils. Supercritical fluid extraction uses pressurized carbon dioxide as a solvent. Continuous extraction methods like Soxhlet extraction involve repeatedly treating plant material with fresh solvent to efficiently extract components. The key factors that influence extraction method selection include the plant composition, solvent characteristics, and temperature effects on solubility and stability of chemical components.