Exploring Substances:
Acidic, Basic, and
Neutral
Welcome to the fascinating world of acids and bases! Join siblings Ashwin and
Keerthi as they explore the colorful world of substances at their school's
National Science Day fair. Their adventure begins with a mysterious white paper
that reveals hidden messages when sprayed with a special liquid.
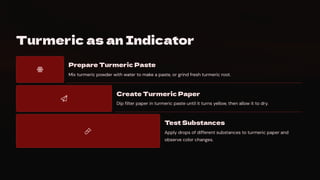
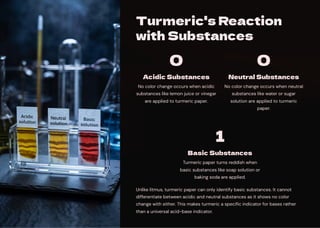
In this presentation, we'll discover how different substances can be classified as
acidic, basic, or neutral. We'll explore natural indicators like litmus, red rose
extract, and turmeric that help us identify these substances through color
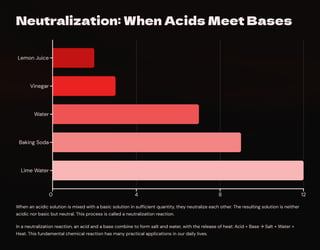
changes. We'll also learn about neutralization reactions and their applications in
our daily lives.
by sandeep swamy