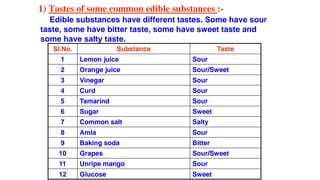
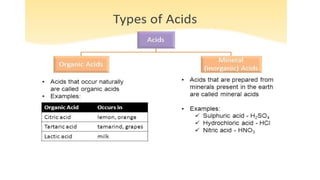
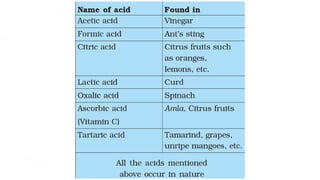
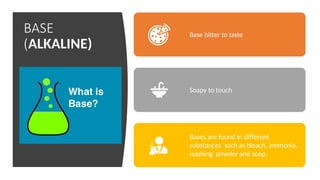
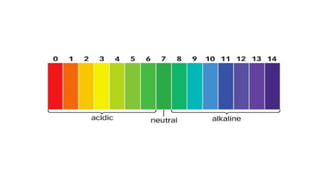
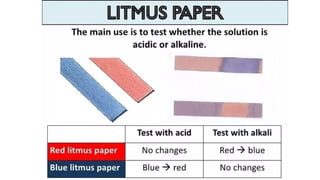
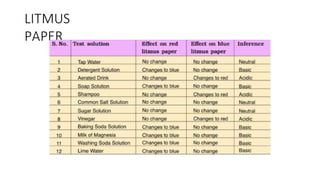
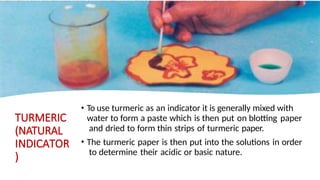
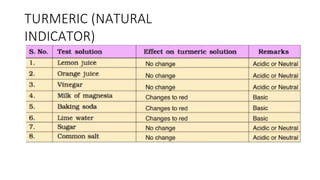
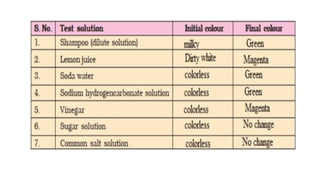
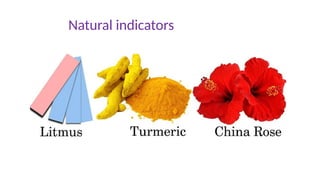
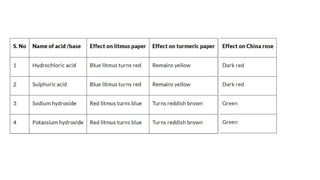
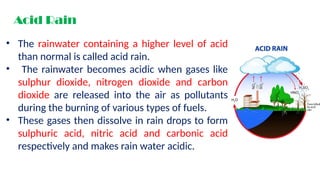
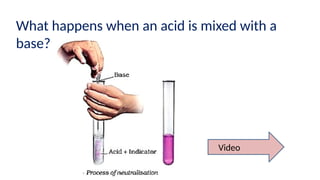
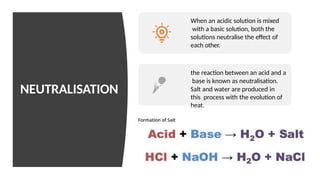
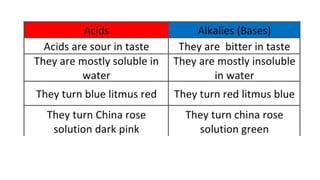
Chapter 4 discusses acids, bases, and neutral substances, defining acids as sour-tasting chemicals and bases as bitter and soapy. It explains the use of indicators like litmus, turmeric, red cabbage, and phenolphthalein for testing acidity and alkalinity, and explores the environmental impact of acid rain. The chapter also covers neutralization reactions between acids and bases, highlighting their applications in everyday life, such as in treating indigestion and soil balance.