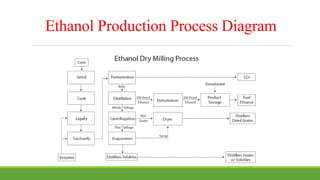
This document summarizes a presentation on ethanol as an alternative fuel for automobiles. It begins with an abstract that outlines the key points to be covered, including ethanol properties, production via dry milling, and advantages/disadvantages as a fuel. The presentation then discusses ethanol as a promising alternative that can replace gasoline due to its cleaner burning properties. It compares ethanol to gasoline properties such as octane number and energy content. India's potential for ethanol production from sugarcane is also reviewed due to its goal of blending 20% ethanol in gasoline by 2017 for increased energy security. Both advantages like reduced emissions and disadvantages like crop land usage are summarized.









![Ethanol Properties & its Comparison with gasoline as
automotive fuel.
Fuel Properties Gasoline Bioethanol
Molecular weight [kg/kmol] 111 46
Density [kg/l] at 15⁰C 0.75 0.80-0.82
Oxygen content [wt-%] 0 34.8
Lower Calorific Value [MJ/kg] at 15ºC 41.3 26.4
Lower Calorific Value [MJ/l] at 15ºC 31 21.2
Octane number (RON) 97 109
Octane number (MON) 86 92
Cetane number 8 11
Stoichiometric AFR [kg air/kg fuel] 14.7 9.0
Boiling temperature [ºC] 30-190 78
Reid Vapour Pressure [kPa] at 15ºC 75 16.5](https://image.slidesharecdn.com/seminar-170921084906/85/Ethanol-A-promising-alternative-fuel-10-320.jpg)