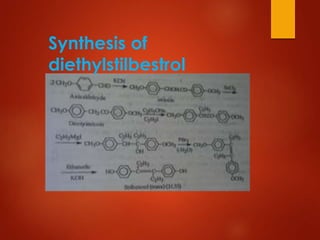
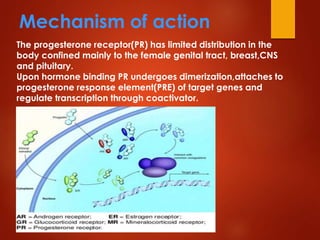
This document provides an overview of estrogen and progestinal agents. It discusses the structure, classes, and mechanisms of action of steroid hormones including estrogens like estradiol, estrone, and estriol as well as progestins. It also covers the synthesis of these compounds, their therapeutic uses for conditions like menopause and contraception, and potential side effects. The key points are that estrogens and progestins are important sex hormones that act through nuclear receptors to regulate gene expression and have applications in hormone replacement therapy and birth control.