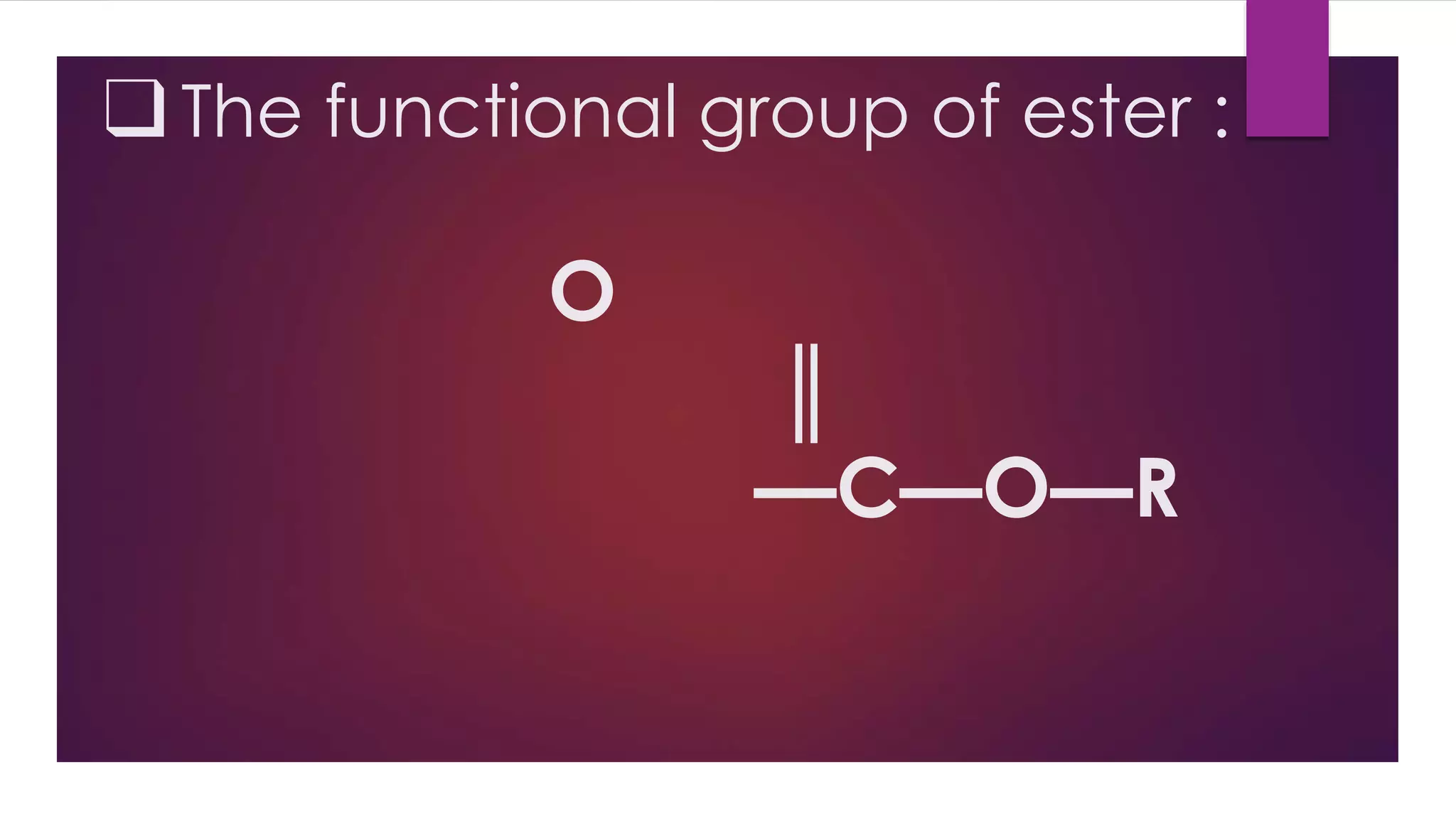
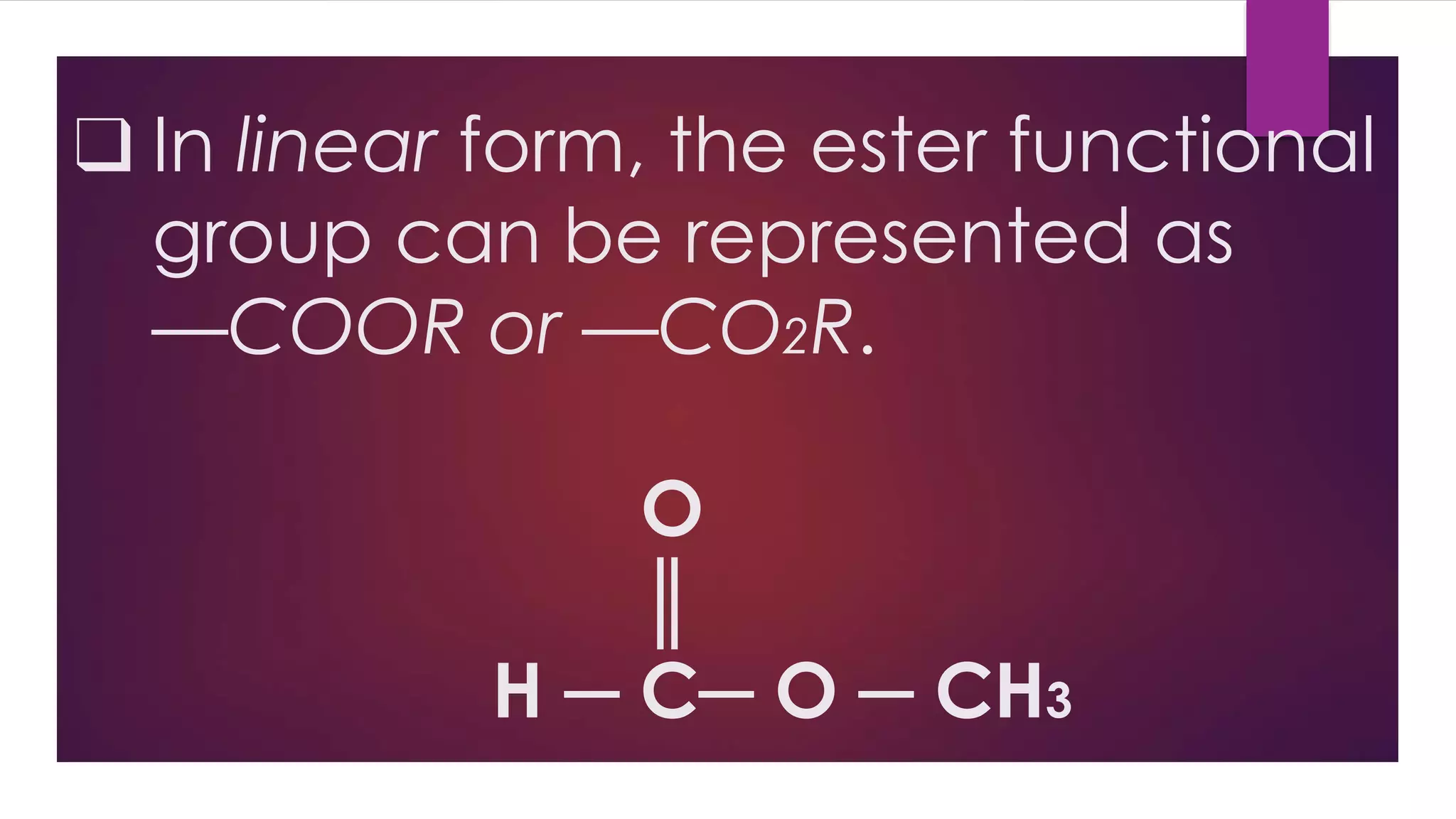
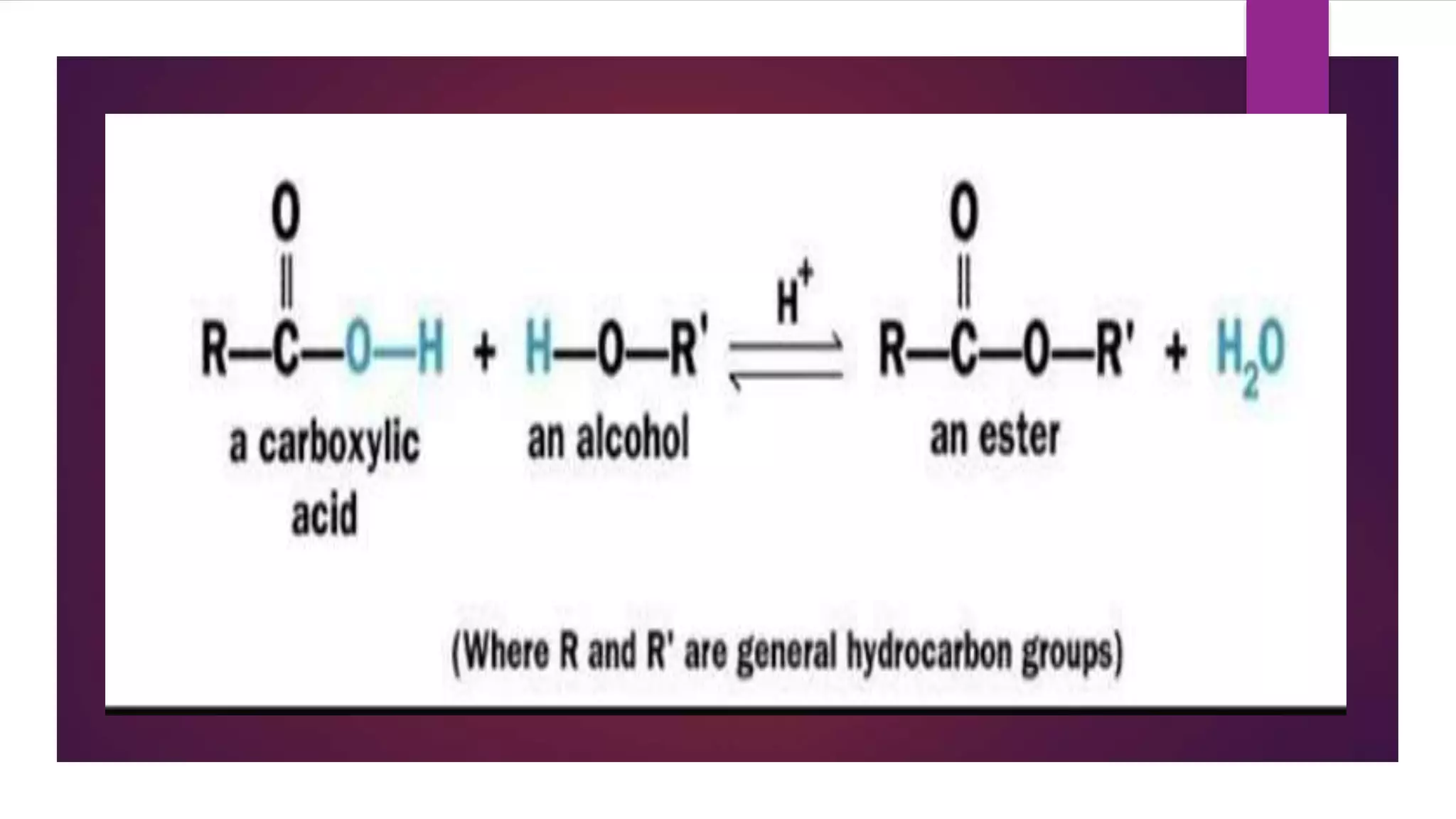
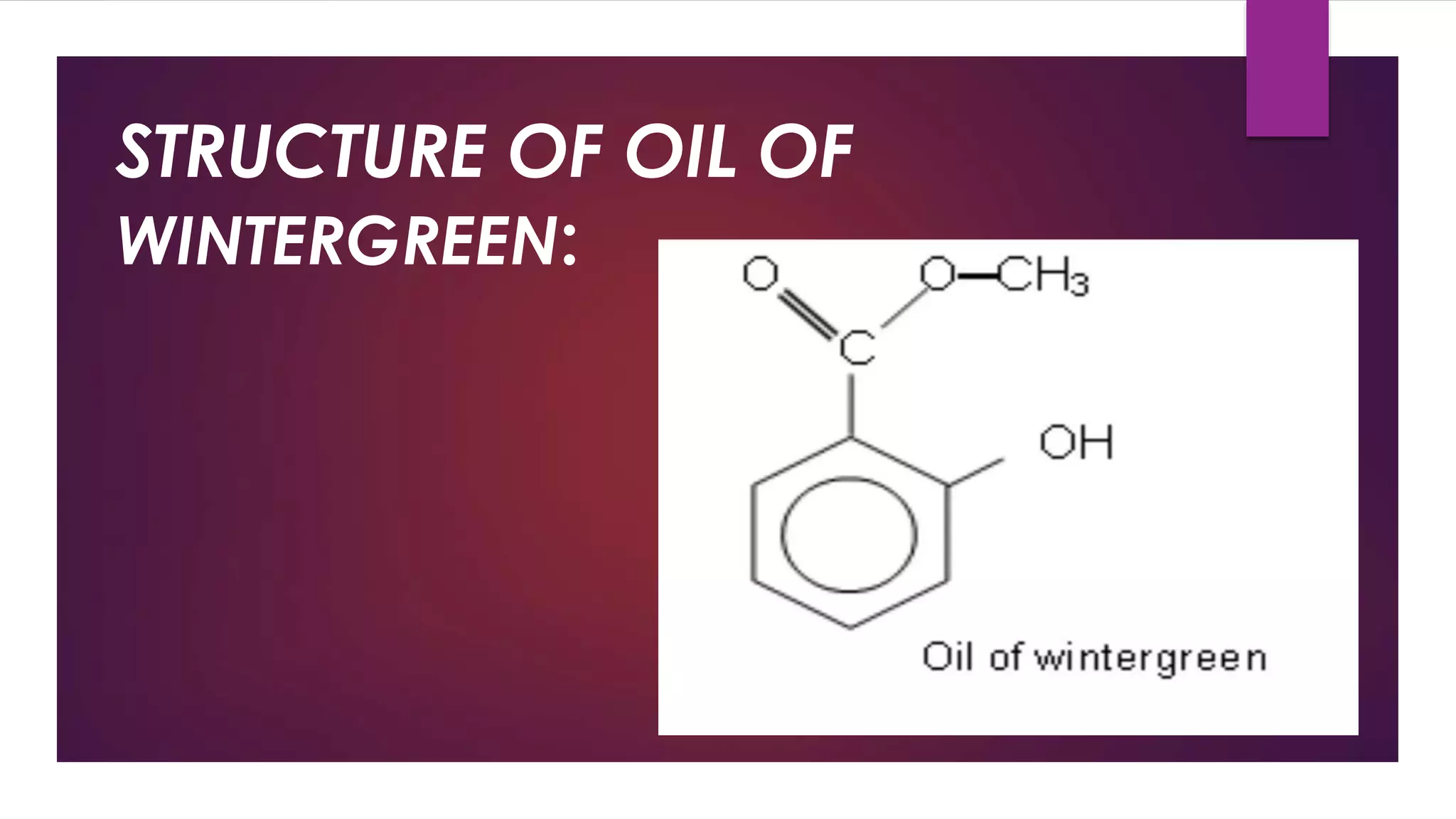
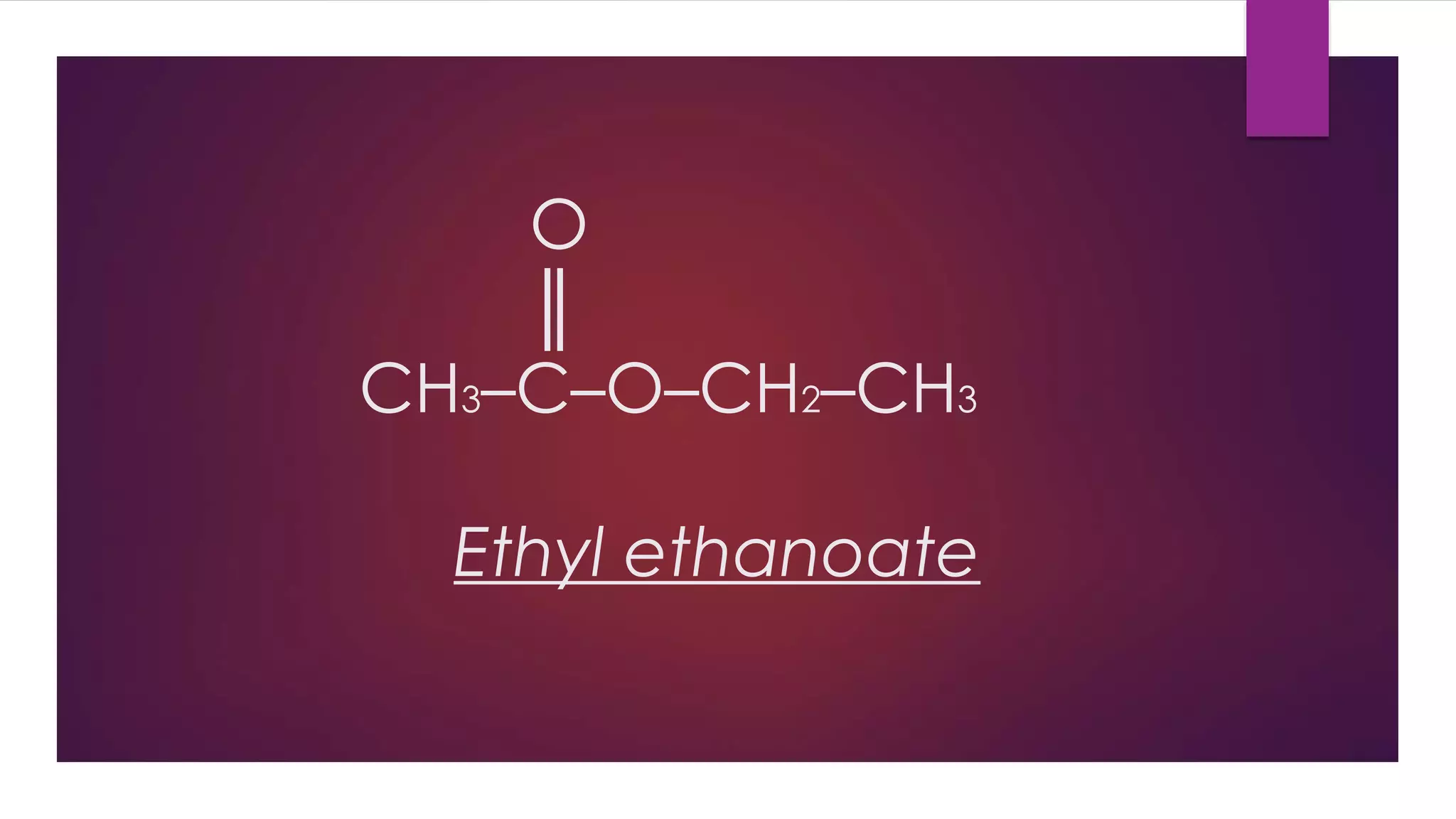
Esters are compounds derived from acids where a hydroxyl group is replaced by an alkoxy group. They have the functional group R-C(=O)-O-R'. Esters are prepared through the reaction of carboxylic acids with alcohols. Their names indicate the parent alcohol and acid. Esters have lower boiling points than the corresponding acids and alcohols. They undergo hydrolysis and saponification reactions. Common esters include benzocaine which is used as a local anesthetic, aspirin which is used as a pain reliever and fever reducer, and oil of wintergreen which contains methyl salicylate used to treat pain.