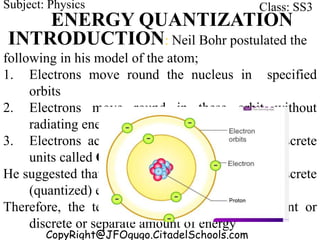
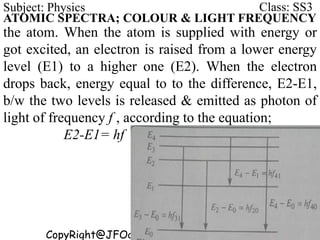
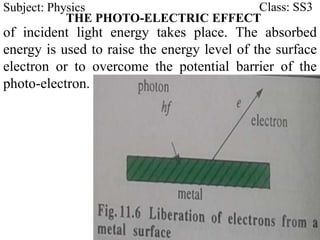
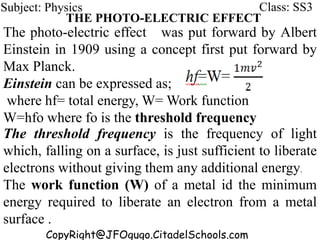
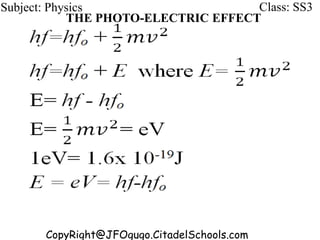
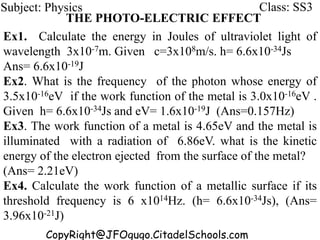
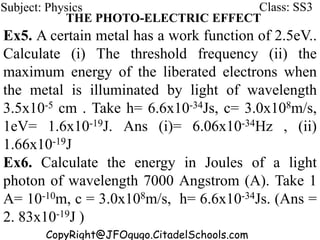
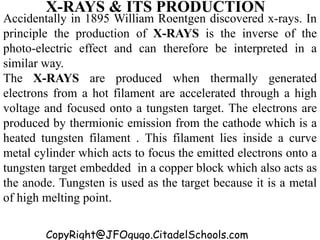
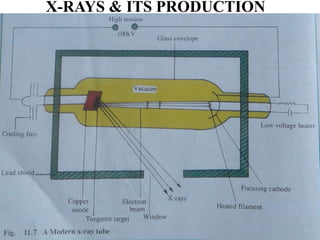
This document discusses the quantization of energy and atomic spectra. It explains that electrons in atoms can only exist in discrete energy levels according to Bohr's model of the atom. When electrons drop from higher to lower energy levels, they emit photons of specific frequencies that are unique to each element, resulting in line spectra. The document also covers Planck's quantum theory of radiation, atomic energy levels, photon emission, the photoelectric effect, and production of x-rays.