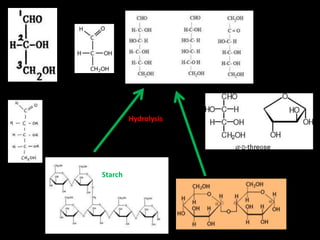
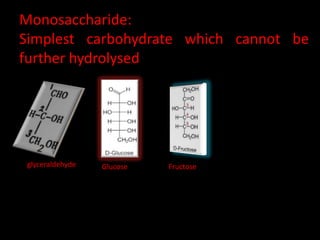
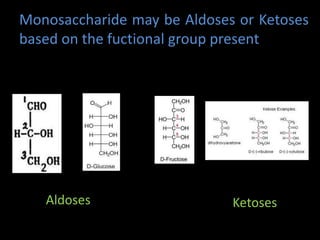
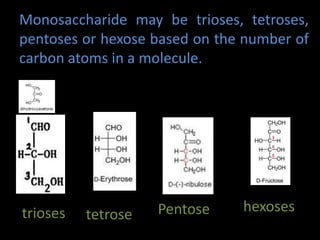
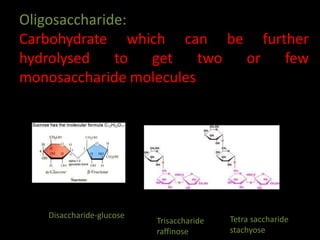
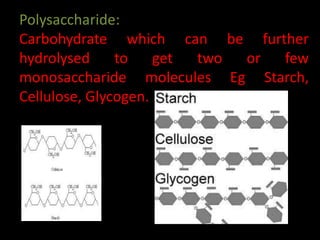
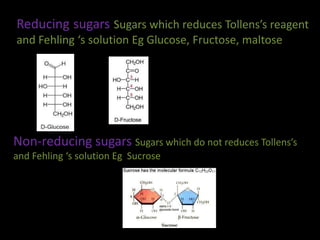
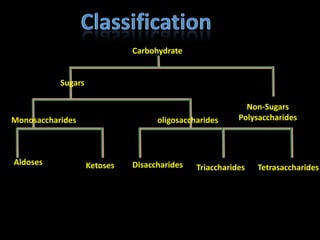
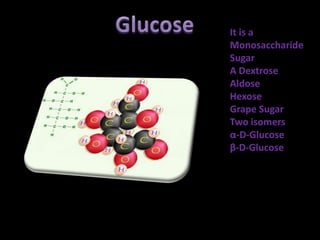
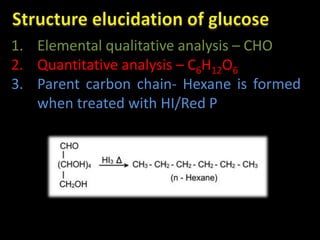
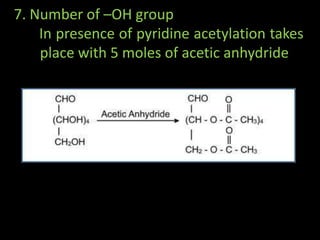
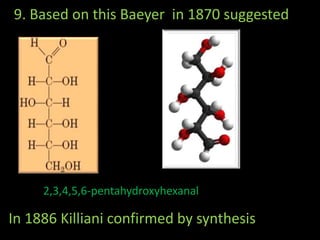
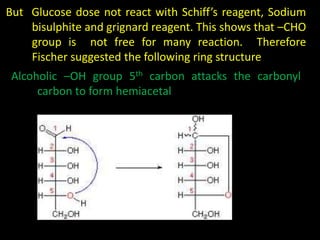
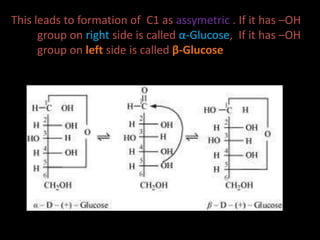
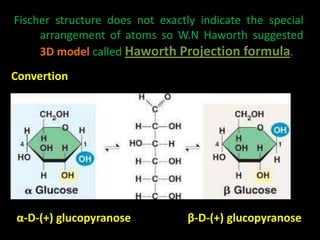
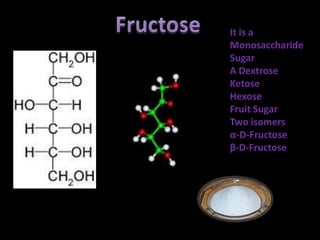
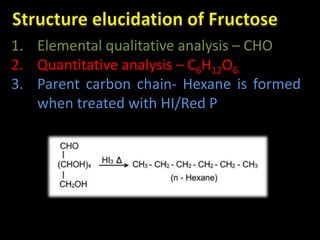
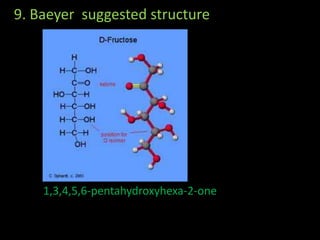
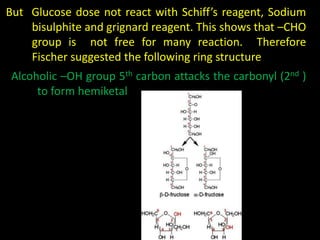
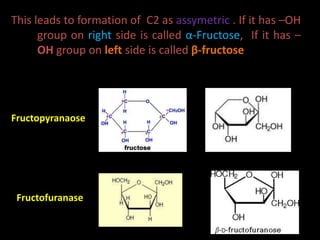
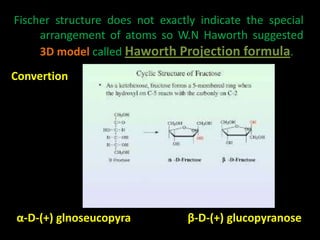
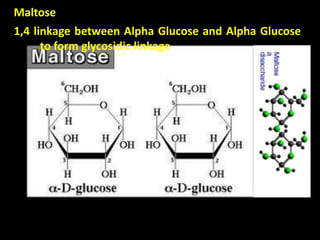
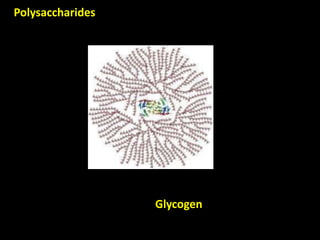
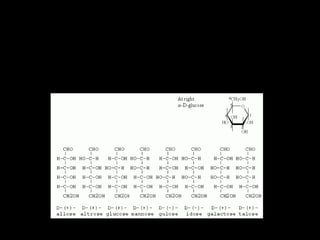
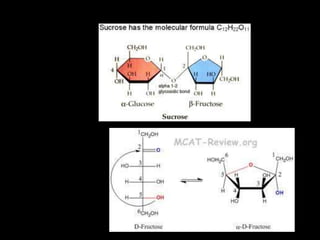
This document discusses carbohydrates, including their classification and properties. It defines carbohydrates as polyhydroxy aldehydes or ketones that produce these compounds upon hydrolysis. Carbohydrates are classified as monosaccharides, oligosaccharides, or polysaccharides depending on the number of monosaccharide units they contain upon hydrolysis. Common monosaccharides include glucose and fructose, which can exist as aldoses or ketoses depending on whether they contain an aldehyde or ketone functional group. The document provides detailed information on the structure, properties, and isomers of glucose and fructose. It also discusses specific di- and polysaccharides like maltose, sucrose, and glycogen.