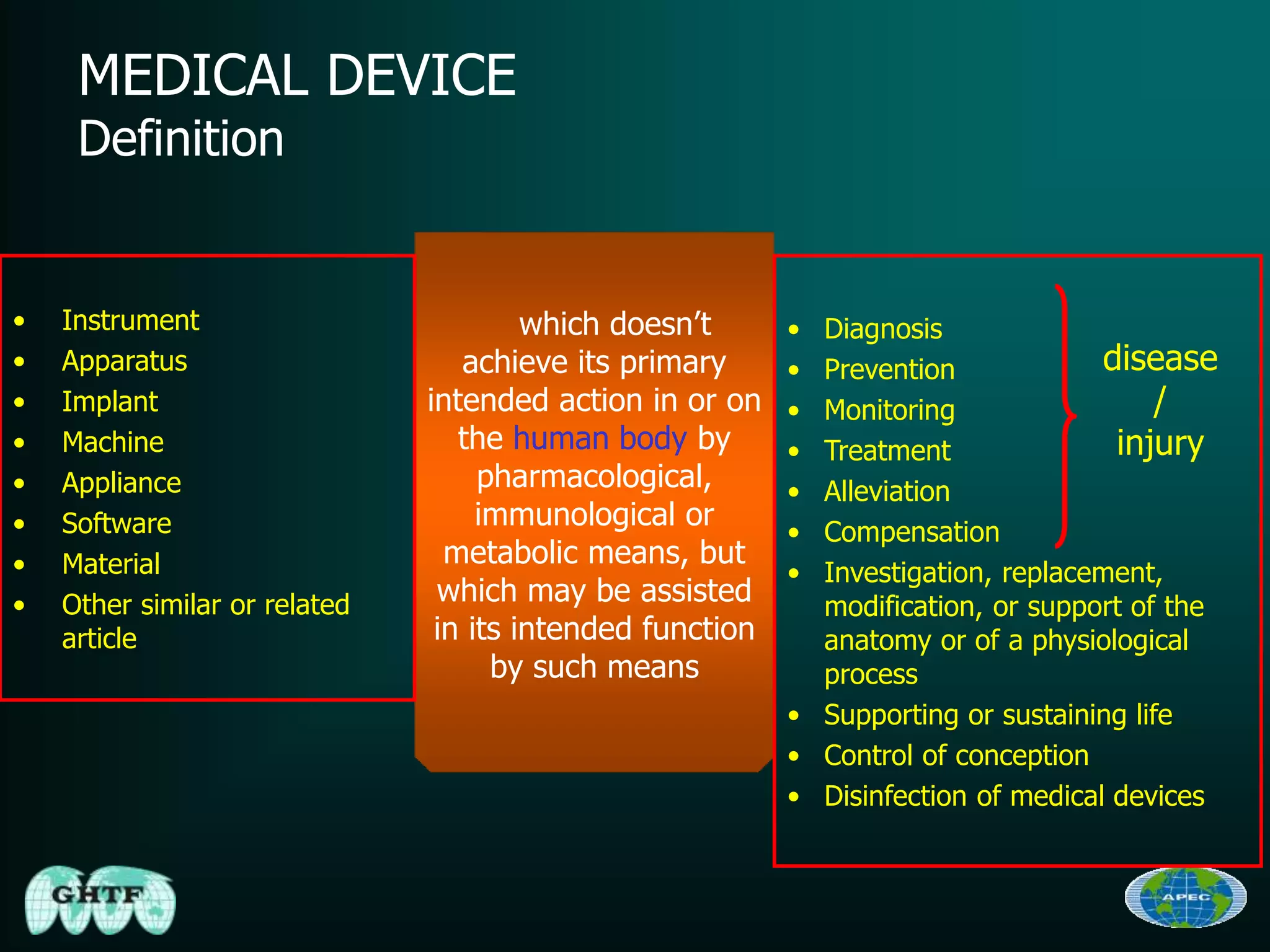
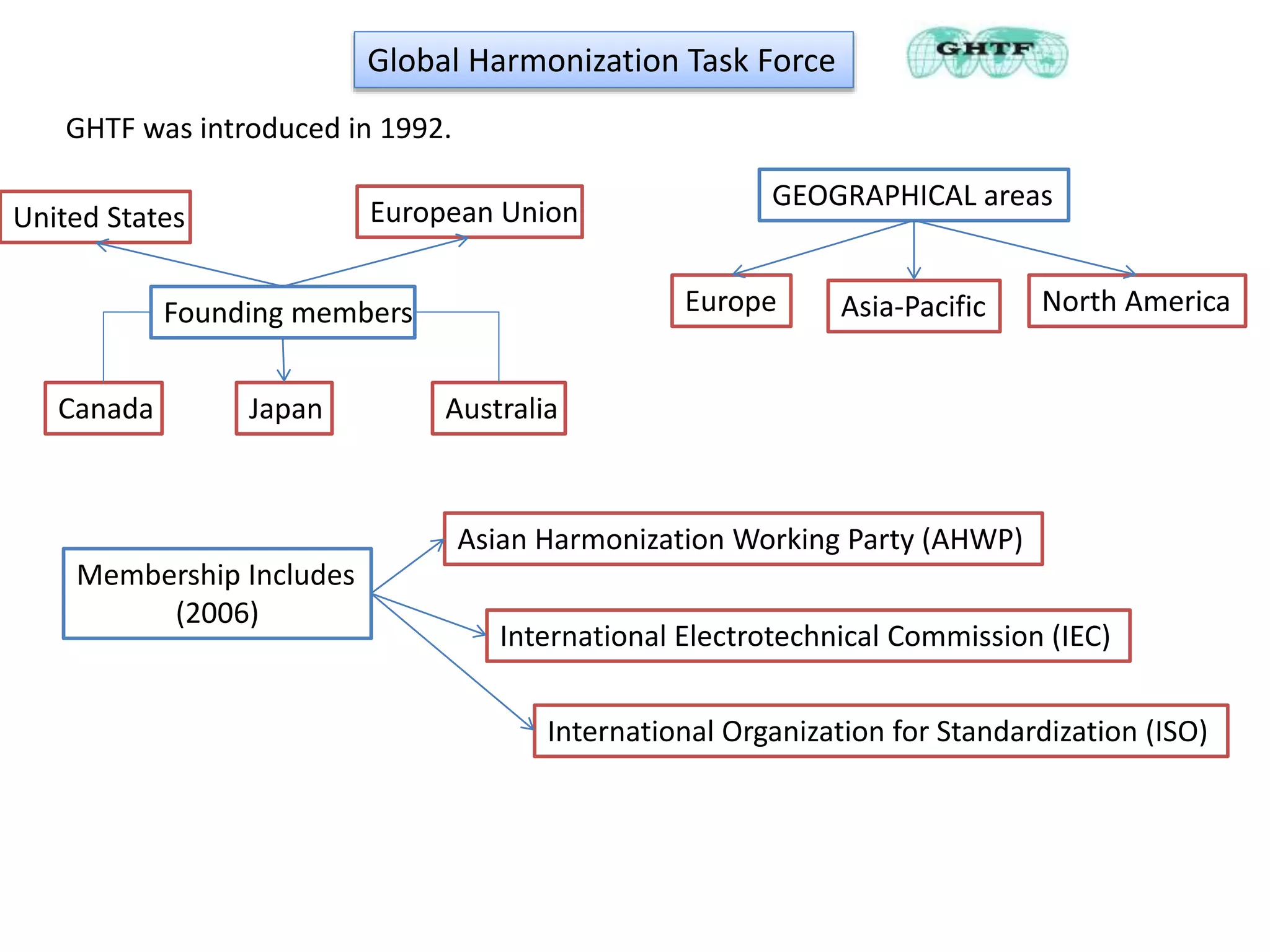
The document discusses the regulatory requirements for importing and authorizing medical devices in the U.S. and Australia, focusing on the approval procedures, particularly for the Medtronic Micra pacemaker. It outlines the classification of medical devices, the importance of compliance with regulations such as the 510(k) process and premarket approval, and the role of various global harmonization bodies. Lastly, it highlights that adherence to these regulatory frameworks is crucial for ensuring the safety and effectiveness of medical devices in the market.










![Global medical device nomenclature
Medical device GMDN code
HIV1/HIV2 antigen IVD, kit, immune-chromatographic test (ICT) rapid. [30832]
Human immunodeficiency viruses (HIV) [CT284]
Viral Infectious disease IVDs [CT355]
Infectious disease IVDs [CT701]
Multiple urine analyte IVD, kit, colorimetric dipstick, rapid [30225]
Urine screening IVDs [CT1246]
Clinical chemistry biological screening IVDs [CT1236]
Clinical chemistry IVDs [CT287]
Glucose monitoring system IVD, home use/point-of-care [30854]GMDN codes
Post-market vigilance information
e-commerce
Medical record keeping
Inventory purposes
Research
GMDN
•GMDN code represents the generic descriptor to internationally standardize device identification.
•It provides secure data exchange between competent authorities and others](https://image.slidesharecdn.com/dan5osedtwoyxnl03lme-signature-c65919d299b75298be909aad8bd20088121c392dad84700702ce0252743bfebe-poli-171016122629/75/Medical-devices-13-2048.jpg)



















![521 CFR [Code of Federal Regulations]
Part 11 of the Title 21 Code of Federal Regulations that establishes the USFDA regulations on
Electronic Records and Electronic Signatures (ERES). Which classifies under SUBCHAPTER H: -
MEDICAL DEVICES PART 870: CARDIOVASCULAR DEVICES; Subpart D: - Cardiovascular Prosthetic
Devices; Sec. 870.3610 Implantable pacemaker pulse generator.
[Title 21, Volume 8]
[Revised as of April 1, 2016]
[CITE: 21CFR870.3610]
TITLE 21: - FOOD AND DRUGS
CHAPTER I: - FOOD AND DRUG
ADMINISTRATION DEPARTMENT OF
HEALTH AND HUMAN SERVICES
SUBCHAPTER H: - MEDICAL DEVICES
PART 870: - CARDIOVASCULAR DEVICES
Subpart D: - Cardiovascular Prosthetic Devices
Sec. 870.3610 Implantable pacemaker pulse
generator.
Micra TM Filing of 21 CFR
Regulatory requirements](https://image.slidesharecdn.com/dan5osedtwoyxnl03lme-signature-c65919d299b75298be909aad8bd20088121c392dad84700702ce0252743bfebe-poli-171016122629/75/Medical-devices-33-2048.jpg)






![FDA Form -3674, Clinical Trials.gov Data Bank ???
Title VIII of the Food and Drug Administration Amendments Act of 2007 (FDAAA) included a
provision that all PMA applications are required to be accompanied by certification that all
applicable clinical trial information has been submitted to the Clinical Trials.gov data bank
Where the Clinical Studies are Stored
Total Product Life Cycle
The Total Product Life Cycle (TPLC) integrates premarket and post market data about medical
devices. It includes data from CDRH databases including Premarket Approvals (PMA), Premarket
Notifications (510[k]), Adverse Events, and Recalls. The TPLC database is refreshed as each of
the individual data sources is updated. The TPLC database provides data by product, or generic
category of device, and not by individual submission or brand name.](https://image.slidesharecdn.com/dan5osedtwoyxnl03lme-signature-c65919d299b75298be909aad8bd20088121c392dad84700702ce0252743bfebe-poli-171016122629/75/Medical-devices-40-2048.jpg)













