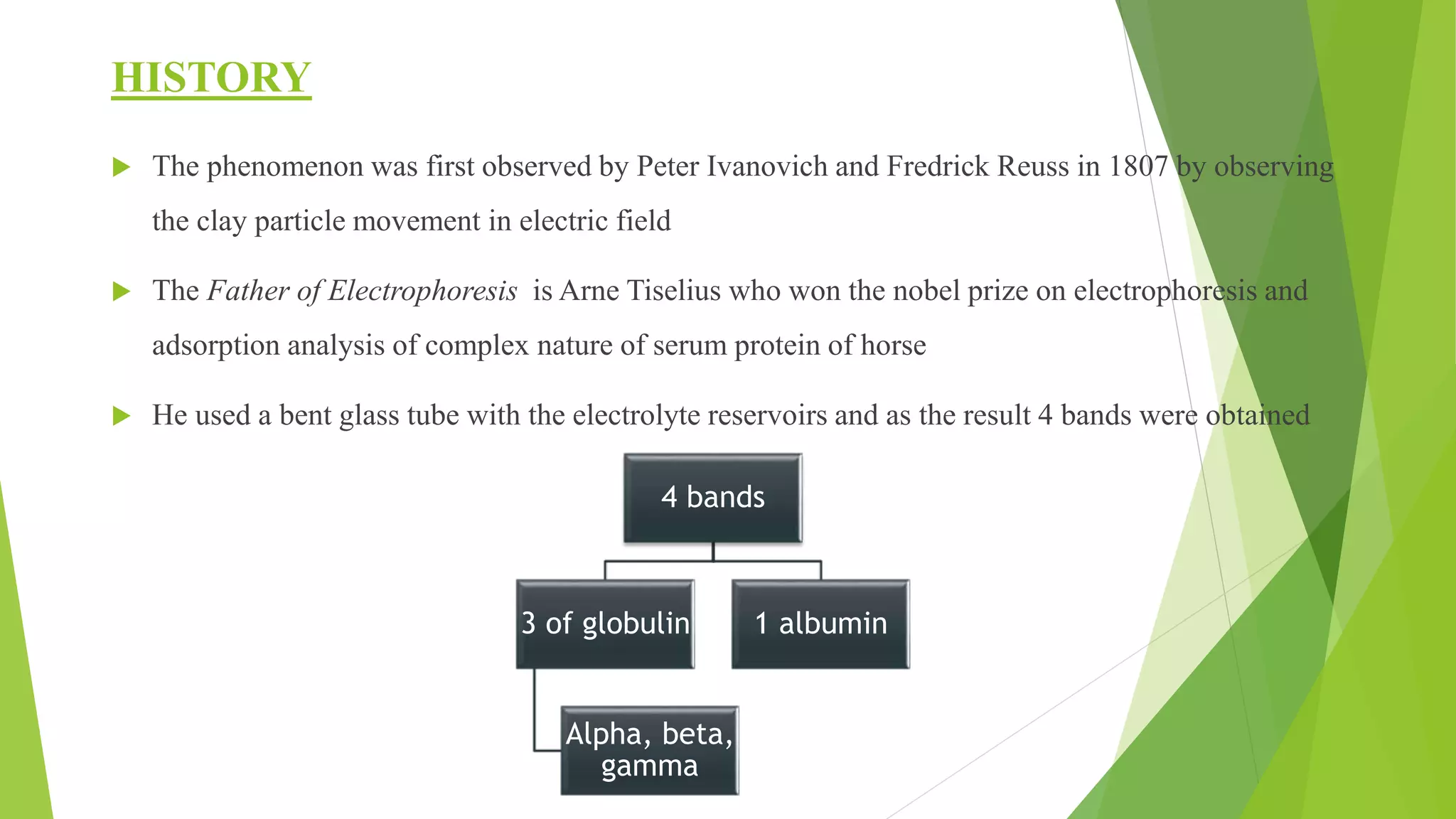
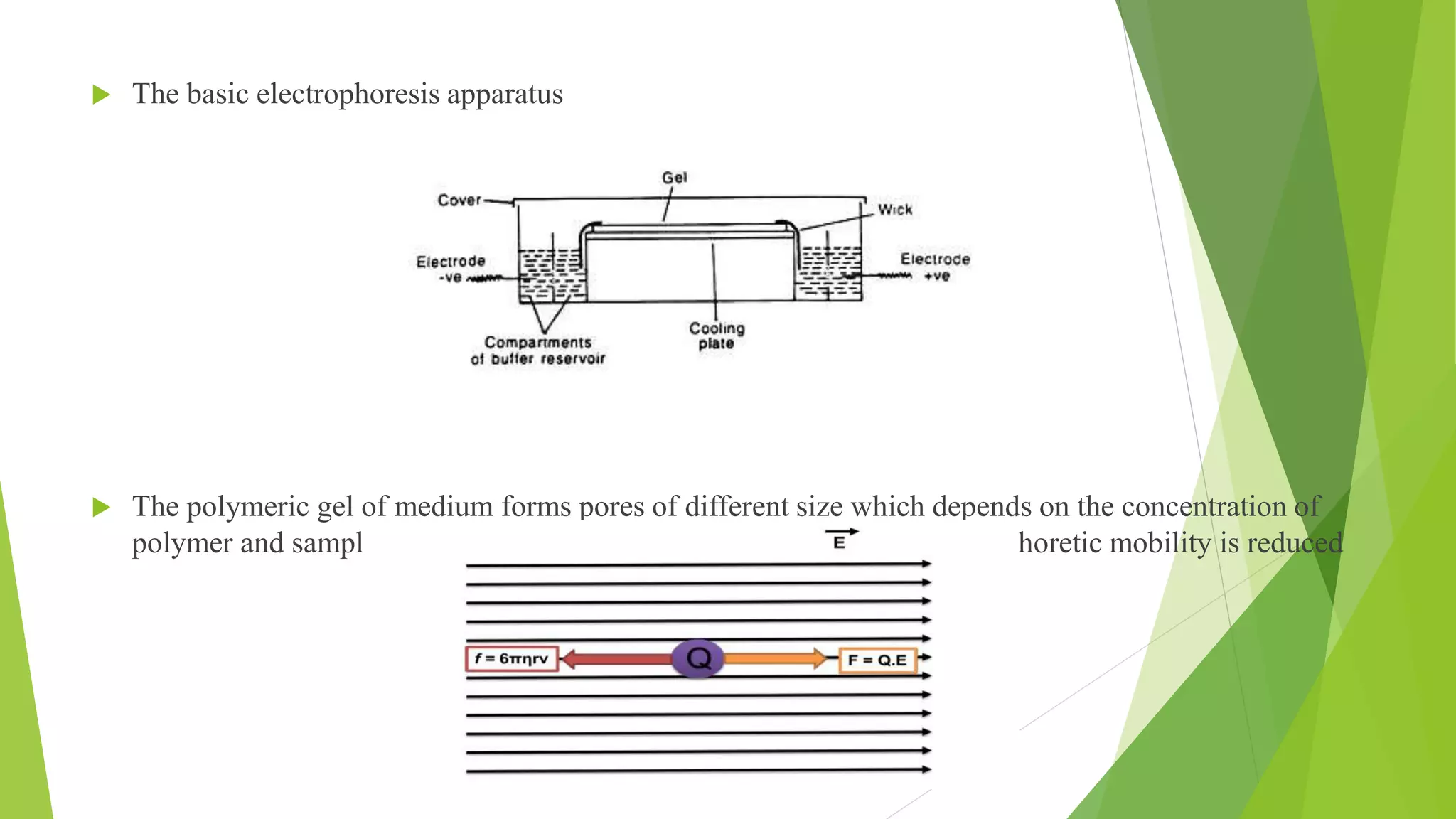
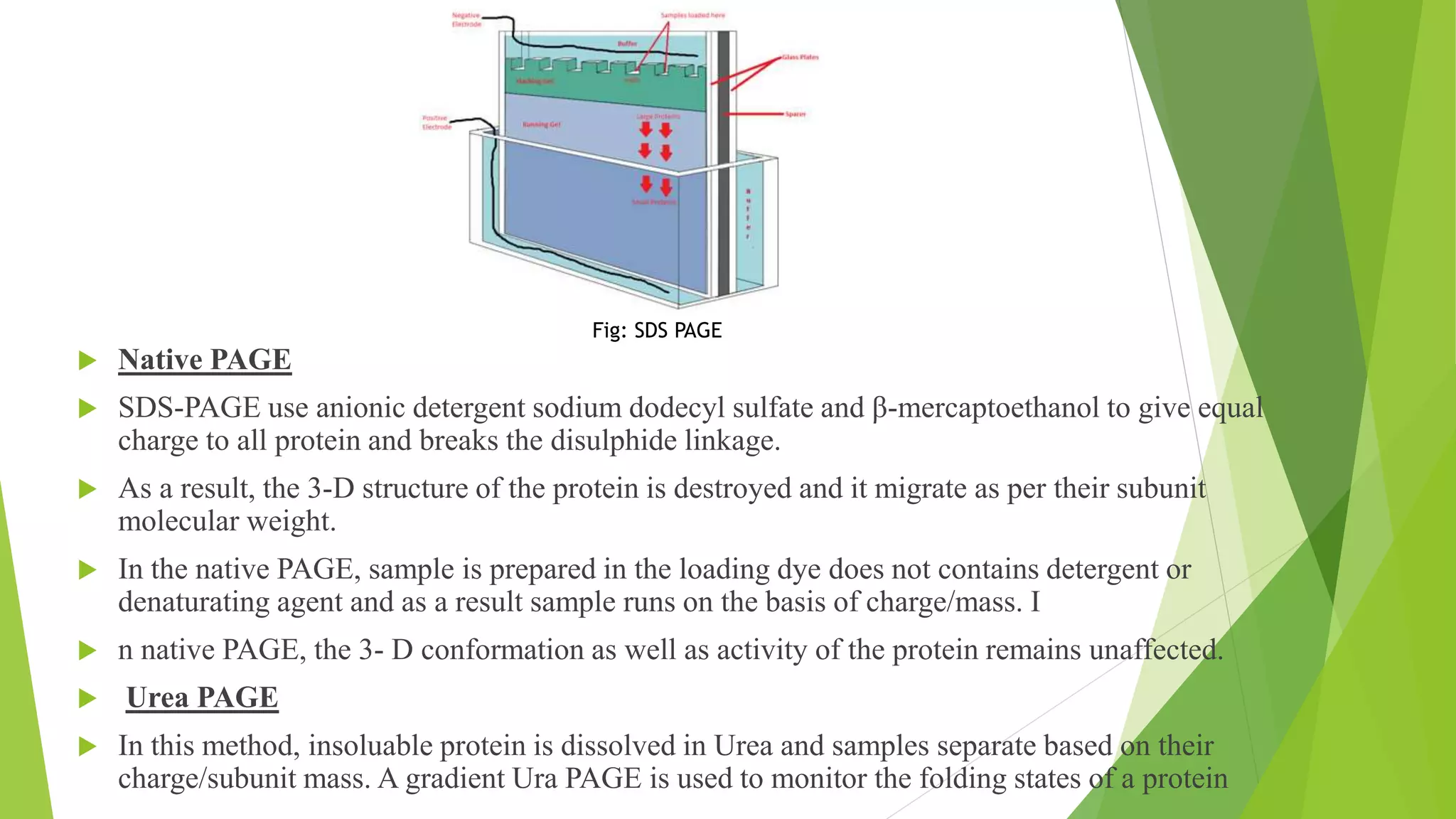
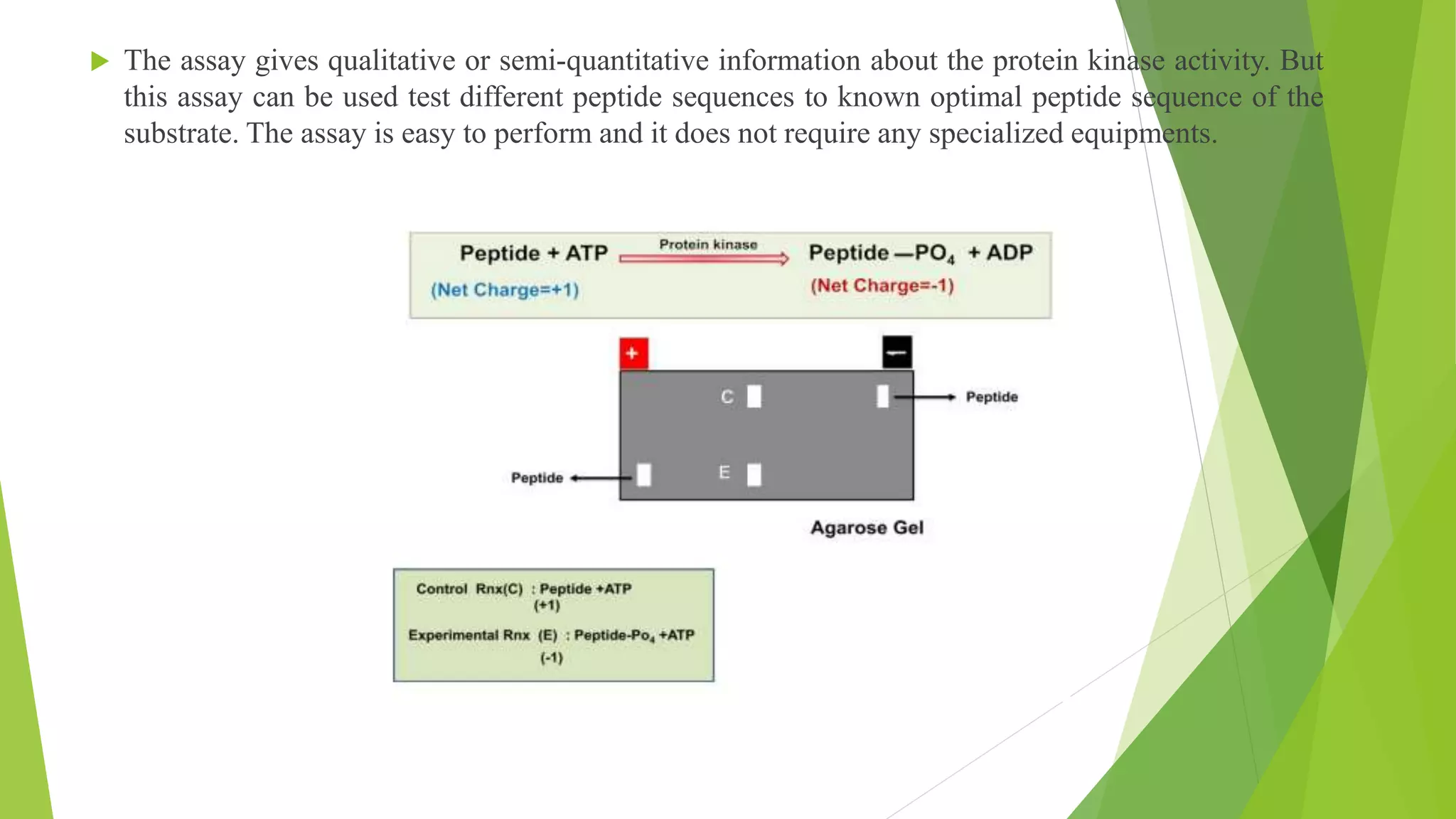
The document provides an in-depth overview of electrophoresis, detailing its principles, history, various types, applications, and the mechanisms involved in the separation of charged particles in an electric field. Key historical figures, such as Arne Tiselius, are mentioned, alongside the explanation of methods like SDS-PAGE and native PAGE, highlighting their specific roles in analyzing biomolecules. It also touches upon related techniques like Southern blotting and protein kinase assays for further biochemical research application.