Embed presentation
Download to read offline


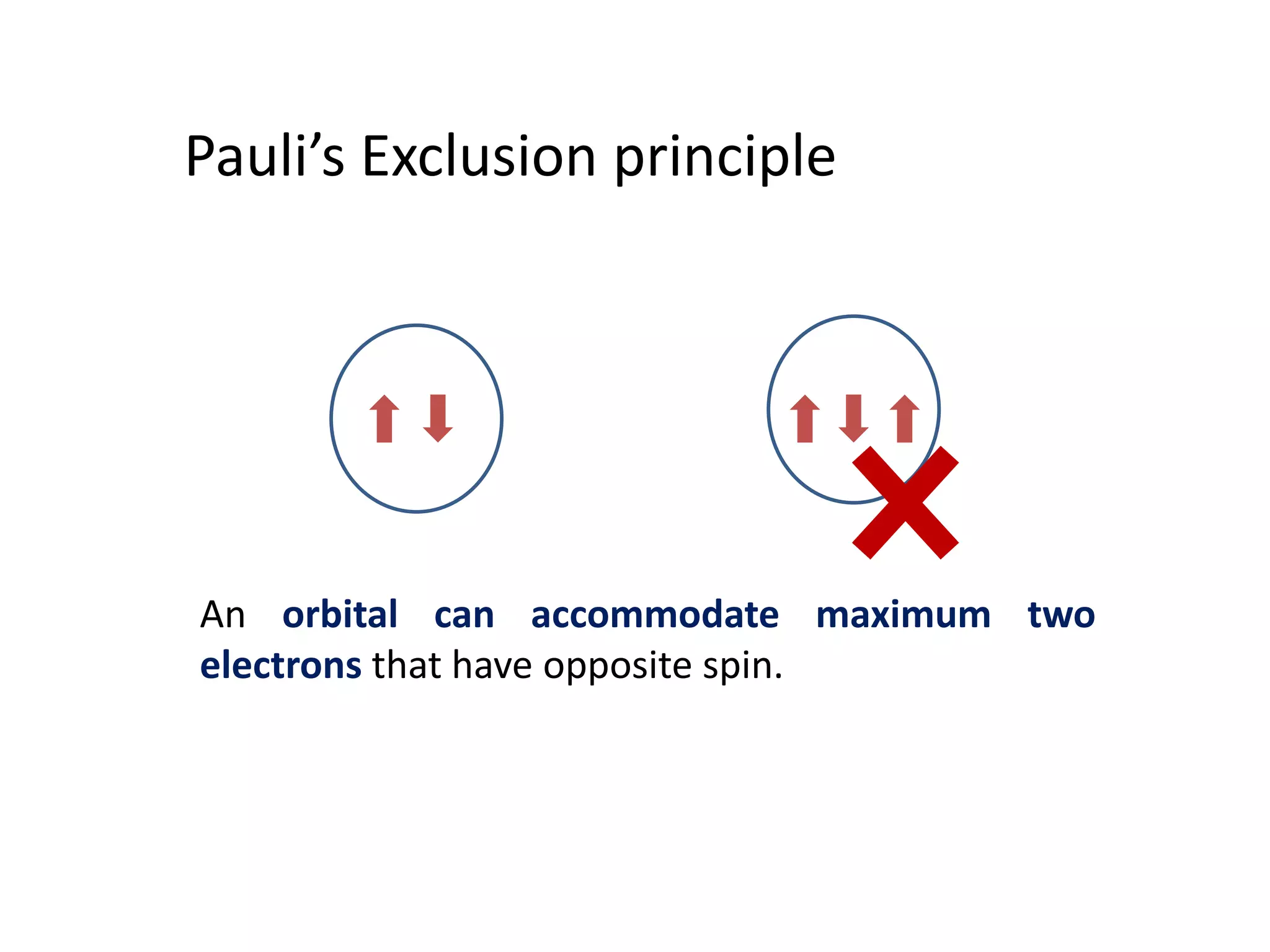
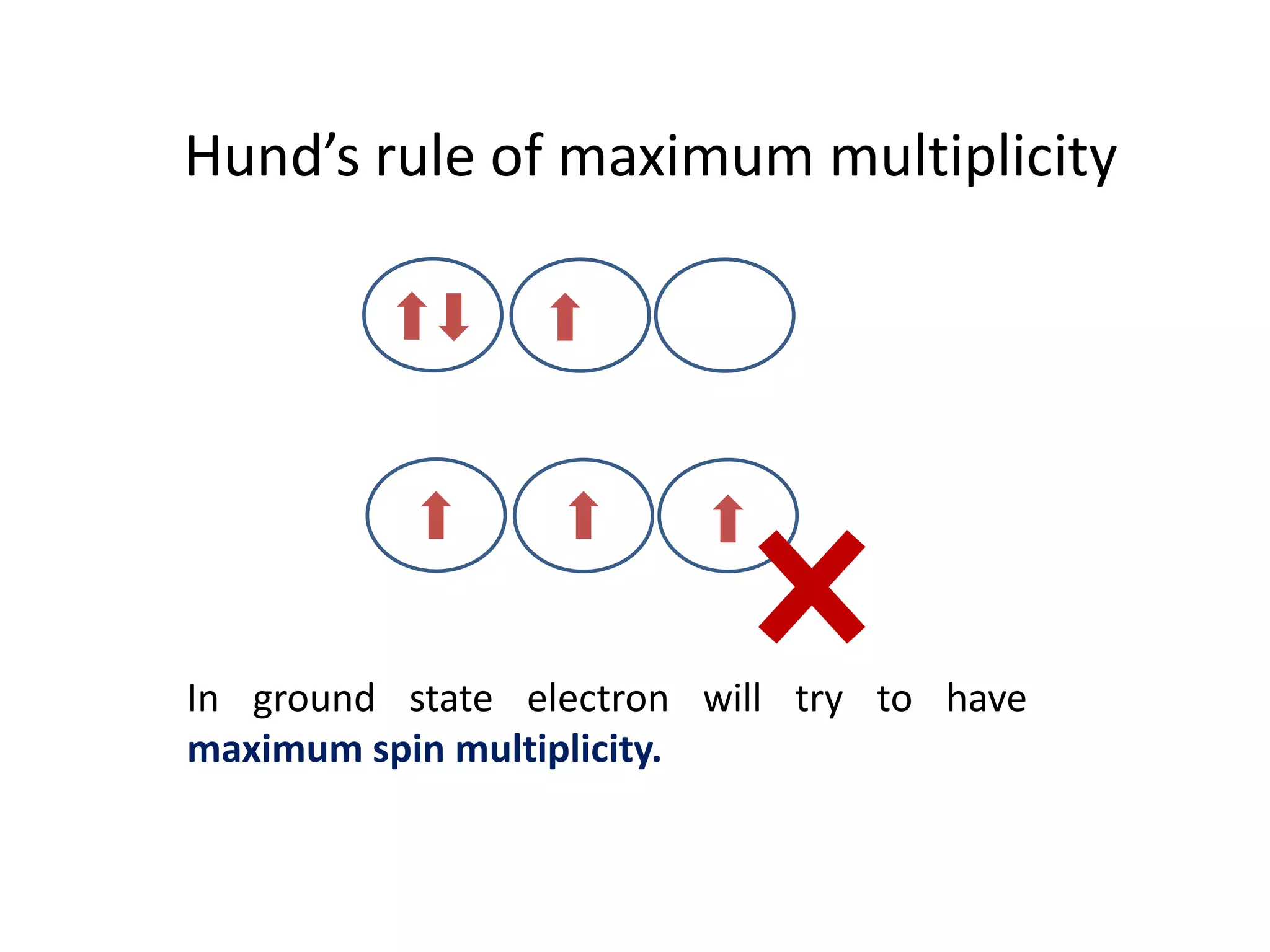
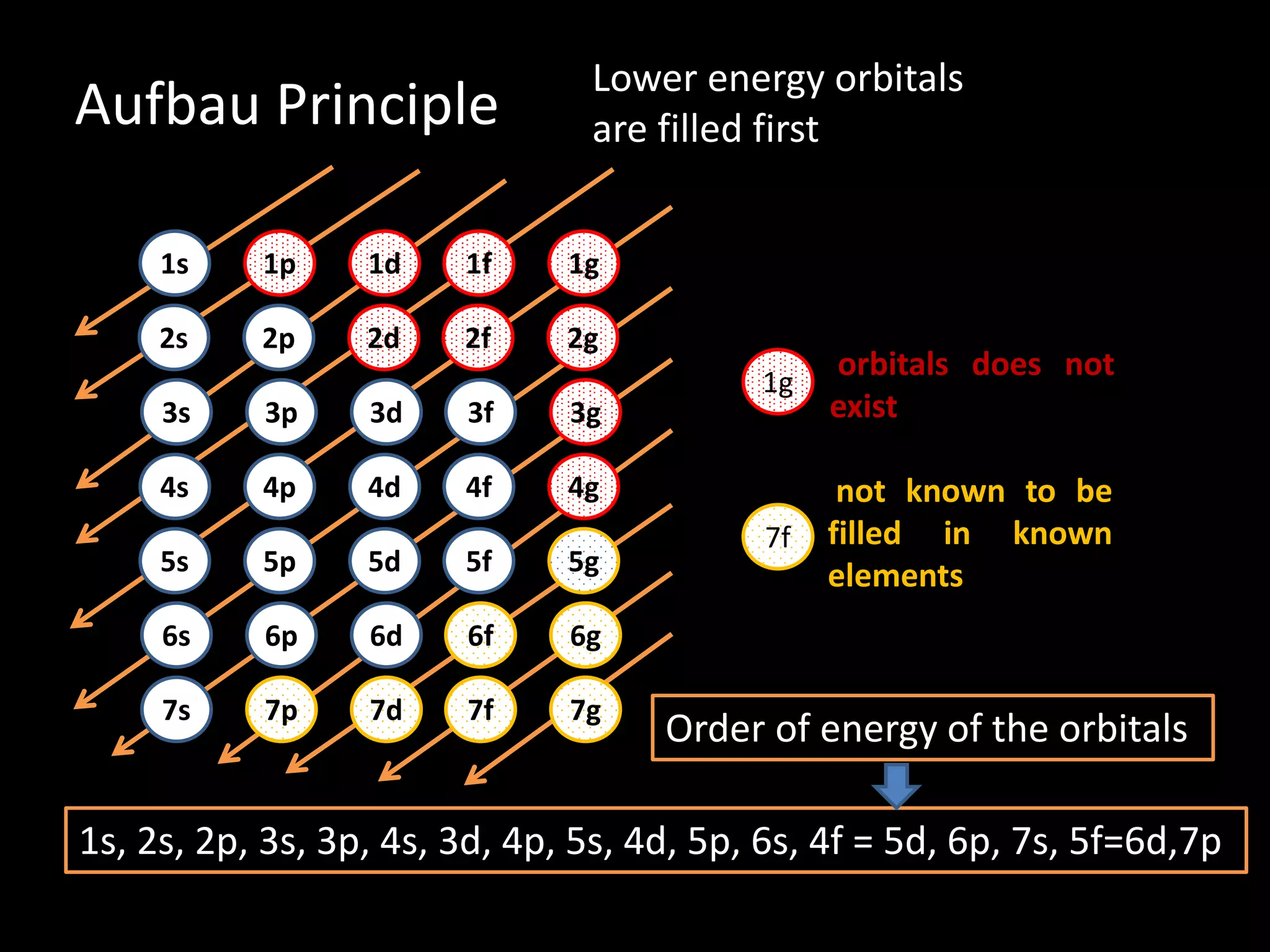
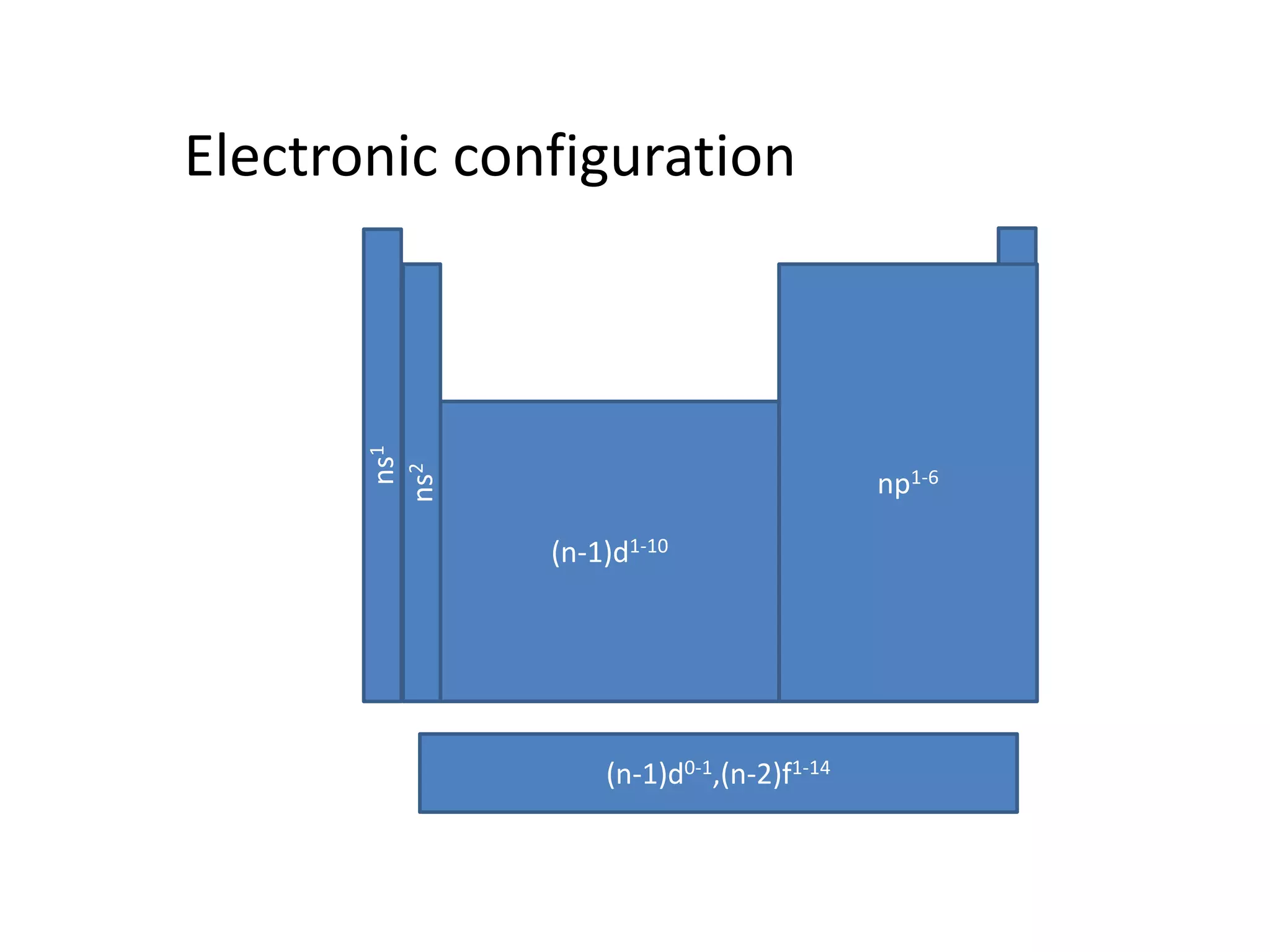
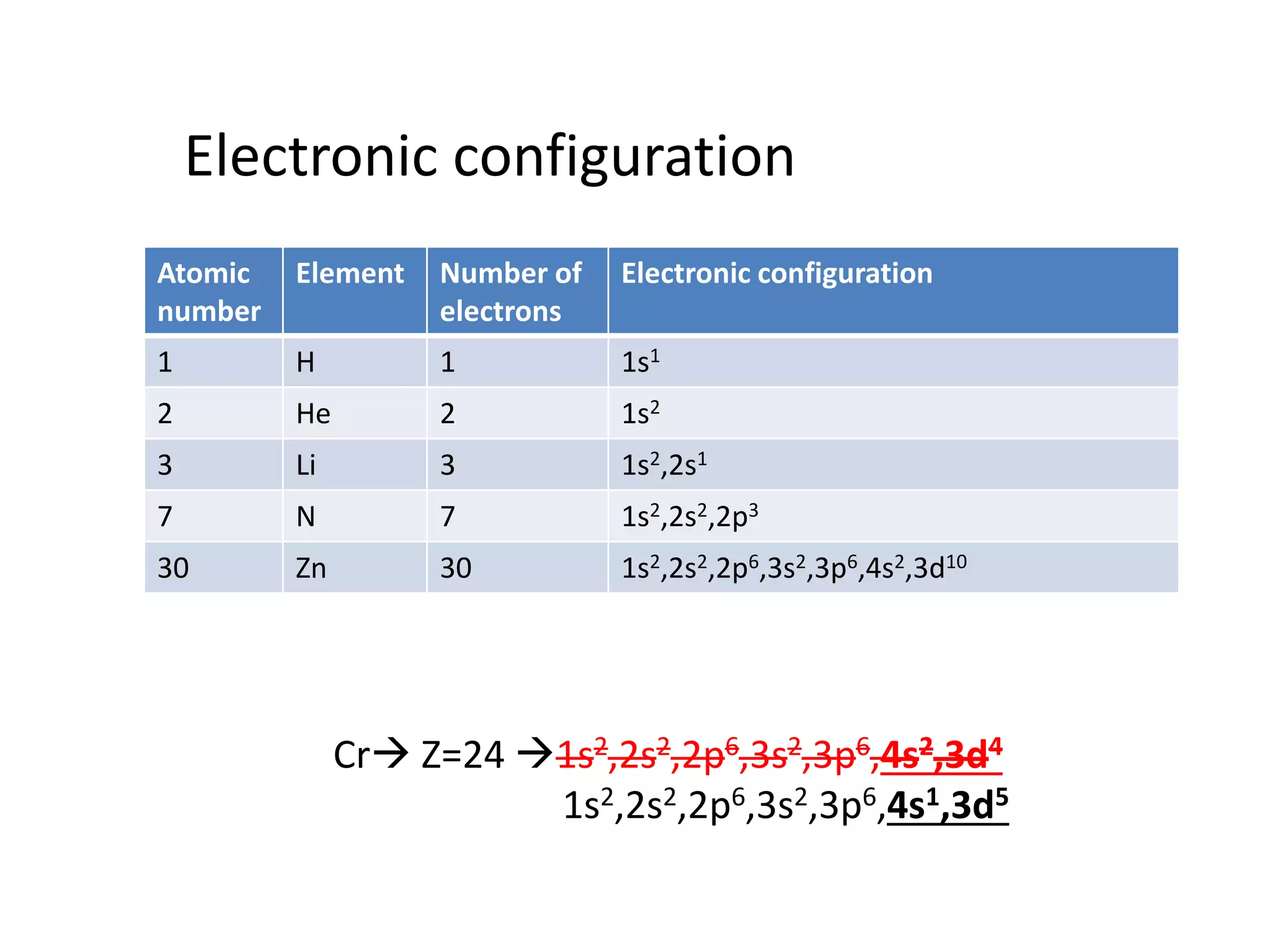
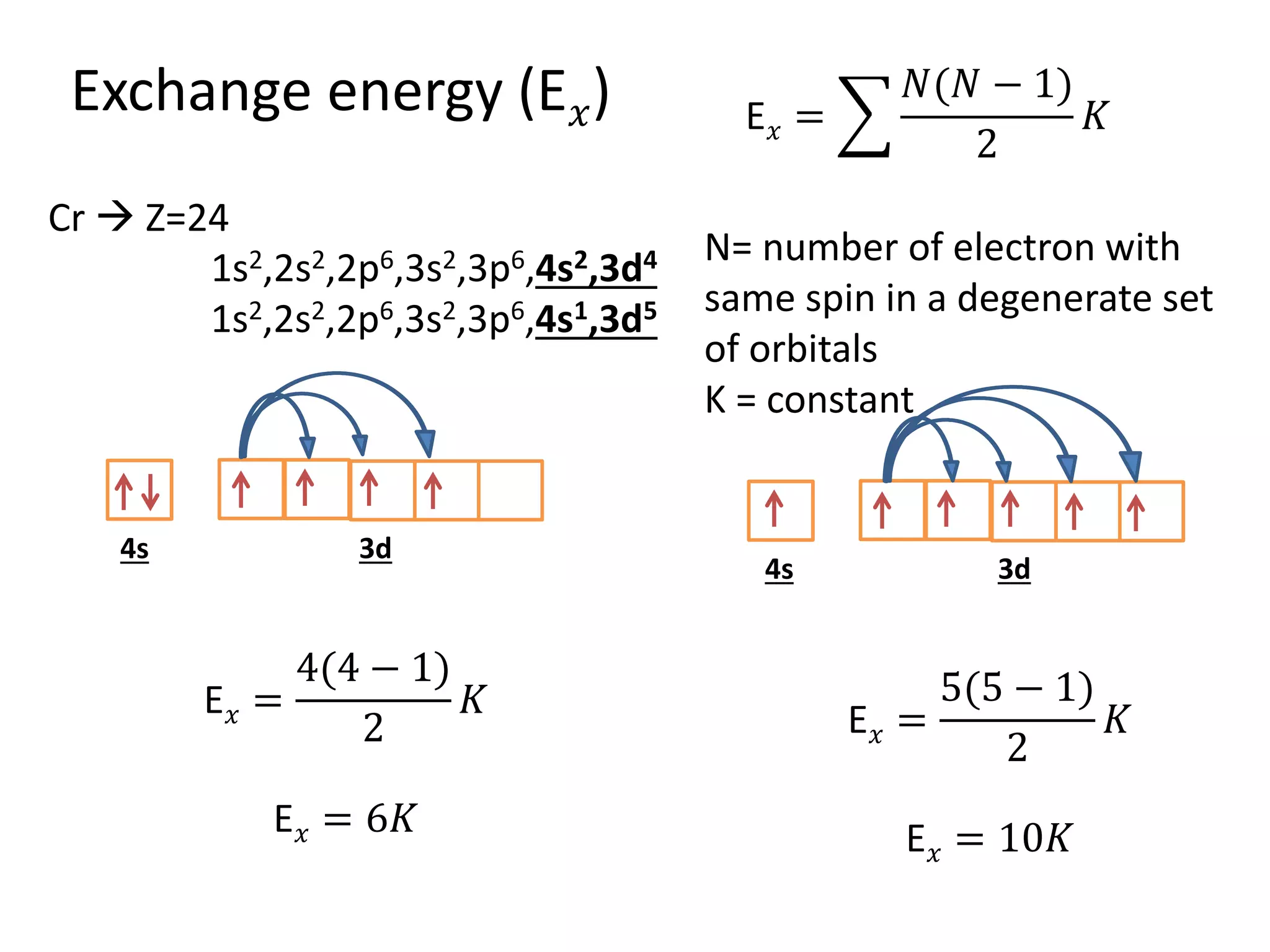
The document discusses the rules for filling electrons in atomic orbitals: - Pauli's exclusion principle states that an orbital can hold a maximum of two electrons with opposite spins. - Hund's rule of maximum multiplicity states that electrons will occupy orbitals to achieve the maximum number of unpaired spins in the ground state. - The Aufbau principle states that electrons fill from the lowest to highest energy orbitals, following the order 1s, 2s, 2p, 3s, 3p, 4s, etc.







