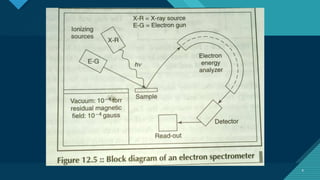
The document discusses various surface spectroscopy techniques including electron spectroscopy and ion spectroscopy. Electron spectroscopy techniques covered are electron spectroscopy for chemical analysis (ESCA) and Auger electron spectroscopy (AES). Ion spectroscopy techniques discussed are secondary ion mass spectroscopy (SIMS) and ion scattering spectroscopy (ISS). The document provides details on the principles, instrumentation and applications of these surface analysis techniques.