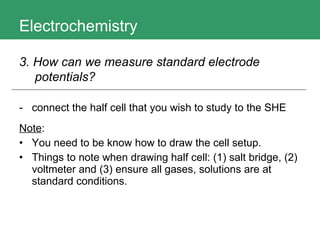
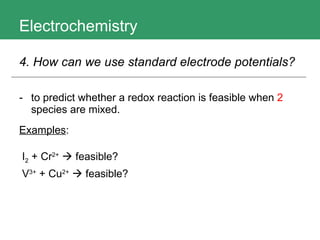
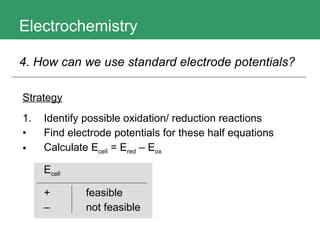
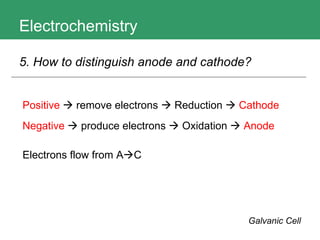
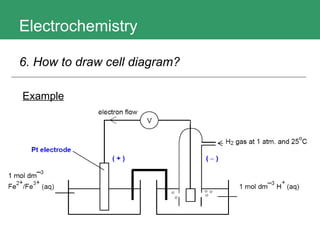
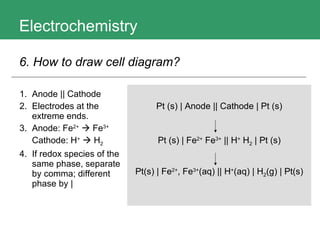
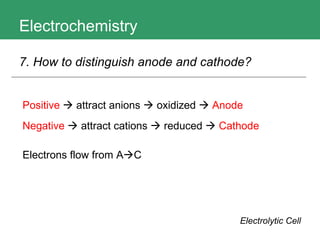
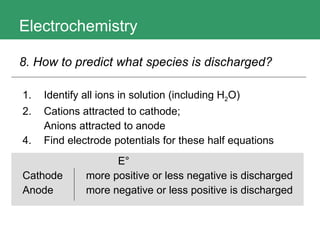
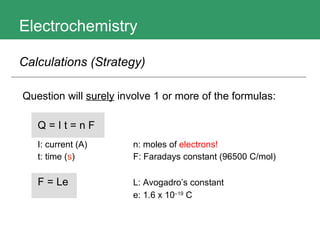
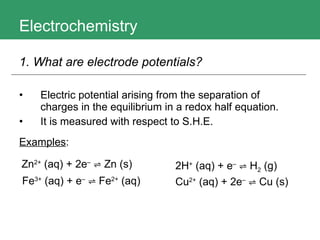
This document discusses key concepts in electrochemistry including electrode potentials, galvanic cells, and electrolytic cells. It defines electrode potentials as the electric potential arising from the separation of charges in redox half reactions. Standard electrode potentials can be measured versus the standard hydrogen electrode and indicate whether the forward or backward reaction is favored. Electrode potentials are also used to predict the feasibility of redox reactions. The document distinguishes anodes and cathodes in galvanic and electrolytic cells and how to draw cell diagrams. It provides strategies for using calculations involving current, time, moles of electrons, and Faraday's constant to solve electrochemistry problems.

![Electrochemistry 2. What can we know from electrode potentials? [Sign] + forward reaction ( reduction ) favoured – backward reaction ( oxidation ) favoured [Magnitude] – the extent the reaction is favoured](https://image.slidesharecdn.com/electrochemistry-110908082317-phpapp01/85/Electrochemistry-5-320.jpg)