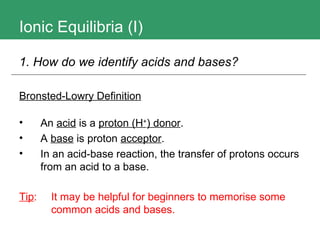
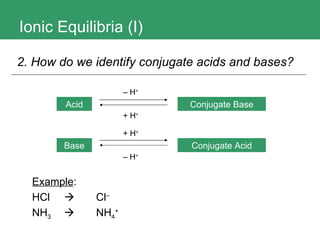
1. The document discusses ionic equilibria, specifically acids and bases, and how to identify and classify them.
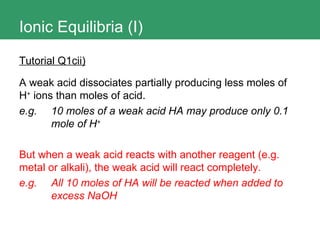
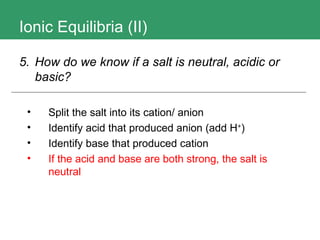
2. It also discusses how to determine if a salt is neutral, acidic, or basic based on the acid and base ions that make it up.
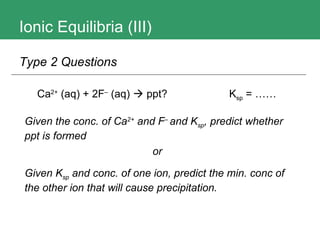
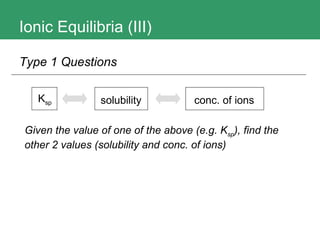
3. The document provides methods for calculating pH and determining precipitation for salt solutions, sparingly soluble salts, and acid-base reactions.




![Ionic Equilibria (I) 3. How do we know whether an acid/ base is strong or weak? Strong acids/ bases ionise completely in aqueous solution. Weak acids/ bases ionise partially in aqueous solution. Tip : An acid/ base can be predicted to be weak when: It is stated so in the question K a or K b value is given Degree/ percent of ionisation is given [H 3 O + ] < [HA]; [OH – ] < [B]](https://image.slidesharecdn.com/ionicequilibria-110908081730-phpapp02/85/Ionic-Equilibria-7-320.jpg)

![Ionic Equilibria (I) 4. How do we calculate pH of an acid/ base? (I) Learn the important terms/ relations pH, pOH K a , pK a K b , pK b K w pH + pOH = 14 pK a + pK b = 14 [H + ][OH – ] = 10 -14 K a . K b = K w = 10 -14](https://image.slidesharecdn.com/ionicequilibria-110908081730-phpapp02/85/Ionic-Equilibria-9-320.jpg)
![Ionic Equilibria (I) 4. How do we calculate pH of an acid/ base? (II) Determine strong or weak Strong acids ionise completely in aqueous solution. [H 3 O + ] = [HA] pH = - log [HA] Strong bases ionise completely in aqueous solution. [OH - ] = [B] pOH = - log [B] Strong Acid Strong Base](https://image.slidesharecdn.com/ionicequilibria-110908081730-phpapp02/85/Ionic-Equilibria-10-320.jpg)
![Ionic Equilibria (I) 4. How do we calculate pH of an acid/ base? (II) Determine strong or weak Weak bases ionise partially in aqueous solution. [OH - ] < [B] pOH = - log [OH – ] Weak Acid Weak Base Weak acids ionise partially in aqueous solution. [H 3 O + ] < [HA] pH = - log [H 3 O + ] [H 3 O + ] = K a × [HA] [OH – ] = K b × [B]](https://image.slidesharecdn.com/ionicequilibria-110908081730-phpapp02/85/Ionic-Equilibria-11-320.jpg)





![Ionic Equilibria (II) Identify ion that hydrolyses H 2 O Write equation of the ion with H 2 O Find pH by treating ion as weak acid or weak base Recall : 6. How do we calculate the pH of a salt solution? [H 3 O + ] = K a × [HA] [OH – ] = K b × [B]](https://image.slidesharecdn.com/ionicequilibria-110908081730-phpapp02/85/Ionic-Equilibria-18-320.jpg)
![Ionic Equilibria (II) Find K a / K b of the ion from K b / K a of the parent acid/ base use K w 2. Find [salt] = 6. How do we calculate the pH of a salt solution? [H 3 O + ] = K a × [HA] [OH – ] = K b × [B] n (limiting reagent) V total V total = V acid + V base](https://image.slidesharecdn.com/ionicequilibria-110908081730-phpapp02/85/Ionic-Equilibria-19-320.jpg)




![Ionic Equilibria (III) CaF 2 (s) ⇌ Ca 2+ (aq) + 2F – (aq) I ? 0 0 C -x +x +2x E ? x 2x Type 1 Questions (Strategy) K sp solubility conc. of ions Expressing each term in x can help in the interconversion. solubility = x K sp = 4x 3 [Ca 2+ ] = x [F – ] = 2x](https://image.slidesharecdn.com/ionicequilibria-110908081730-phpapp02/85/Ionic-Equilibria-25-320.jpg)