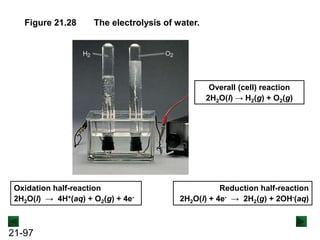
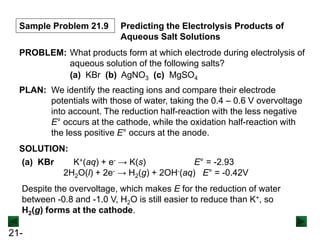
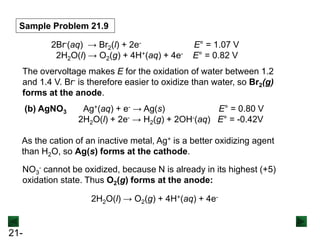
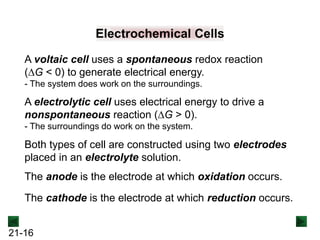
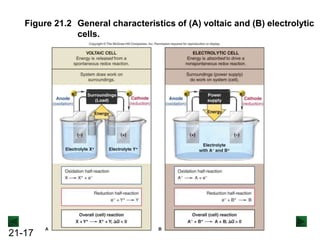
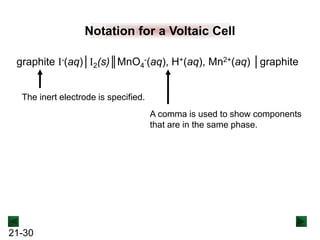
This document provides an overview of electrochemistry and voltaic cells. It discusses redox reactions, how to balance redox reactions using the half-reaction method, and the components and operation of voltaic cells. Specifically, it explains that a voltaic cell uses a spontaneous redox reaction to generate electrical energy by separating the oxidation and reduction half-reactions into two half-cells connected by an external circuit and salt bridge. Electrons flow from the anode, where oxidation occurs, through the external circuit to the cathode, where reduction occurs.






![21-12
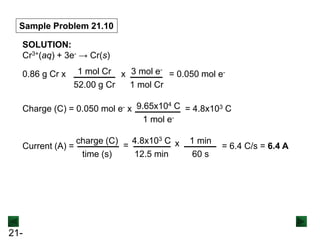
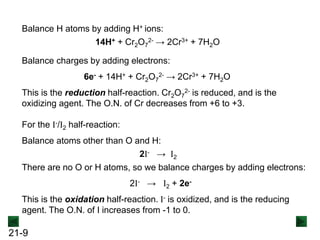
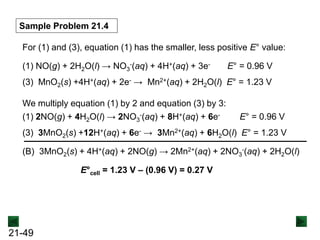
Sample Problem 21.1 Balancing a Redox Reaction in Basic
Solution
PROBLEM: Permanganate ion reacts in basic solution with oxalate
ion to form carbonate ion and solid manganese dioxide.
Balance the skeleton ionic equation for the reaction
between NaMnO4 and Na2C2O4 in basic solution:
MnO4
-(aq) + C2O4
2-(aq) → MnO2(s) + CO3
2-(aq) [basic solution]
PLAN: We follow the numbered steps as described in the text, and
proceed through step 4 as if this reaction occurs in acidic
solution. Then we add the appropriate number of OH- ions
and cancel excess H2O molecules.
SOLUTION:
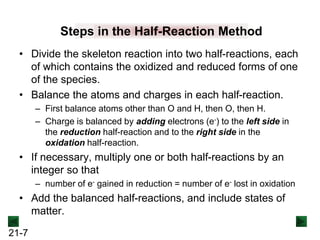
Step 1: Divide the reaction into half-reactions.
MnO4
- → MnO2 C2O4
2- → CO3
2-](https://image.slidesharecdn.com/ch21electrochem6efinal-210505161609/85/Ch21-electrochem-6e_final-12-320.jpg)
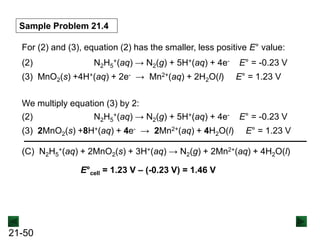
![21-13
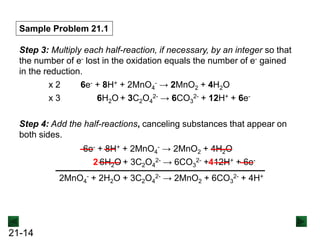
Sample Problem 21.1
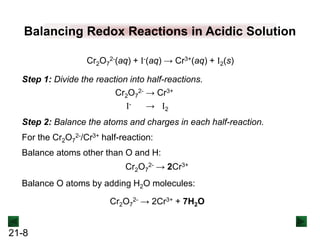
Step 2: Balance the atoms and charges in each half-reaction.
Balance atoms other than O and H:
Balance O atoms by adding H2O molecules:
MnO4
- → MnO2 C2O4
2- → 2CO3
2-
MnO4
- → MnO2 + 2H2O 2H2O + C2O4
2- → 2CO3
2-
Balance H atoms by adding H+ ions:
4H+ + MnO4
- → MnO2 + 2H2O 2H2O + C2O4
2- → 2CO3
2- + 4H+
Balance charges by adding electrons:
3e- + 4H+ + MnO4
- → MnO2 + 2H2O
[reduction]
2H2O + C2O4
2- → 2CO3
2- + 4H+ + 2e-
[oxidation]](https://image.slidesharecdn.com/ch21electrochem6efinal-210505161609/85/Ch21-electrochem-6e_final-13-320.jpg)
![21-15
2MnO4
- + 2H2O + 3C2O4
2- + 4OH- → 2MnO2 + 6CO3
2- + 4H2O
Sample Problem 21.1
Basic. Add OH- to both sides of the equation to neutralize H+, and
cancel H2O.
2MnO4
- + 2H2O + 3C2O4
2- + 4OH- → 2MnO2 + 6CO3
2- + [4H+ + 4OH-]
2
Including states of matter gives the final balanced equation:
2MnO4
-(aq) + 3C2O4
2-(aq) + 4OH-(aq) → 2MnO2(s) + 6CO3
2-(aq) + 2H2O(l)](https://image.slidesharecdn.com/ch21electrochem6efinal-210505161609/85/Ch21-electrochem-6e_final-15-320.jpg)
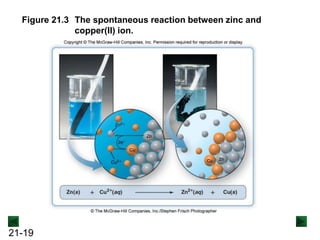
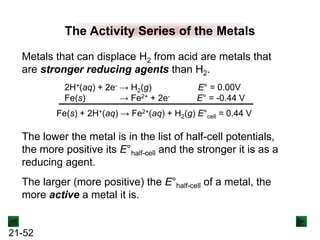
![21-18
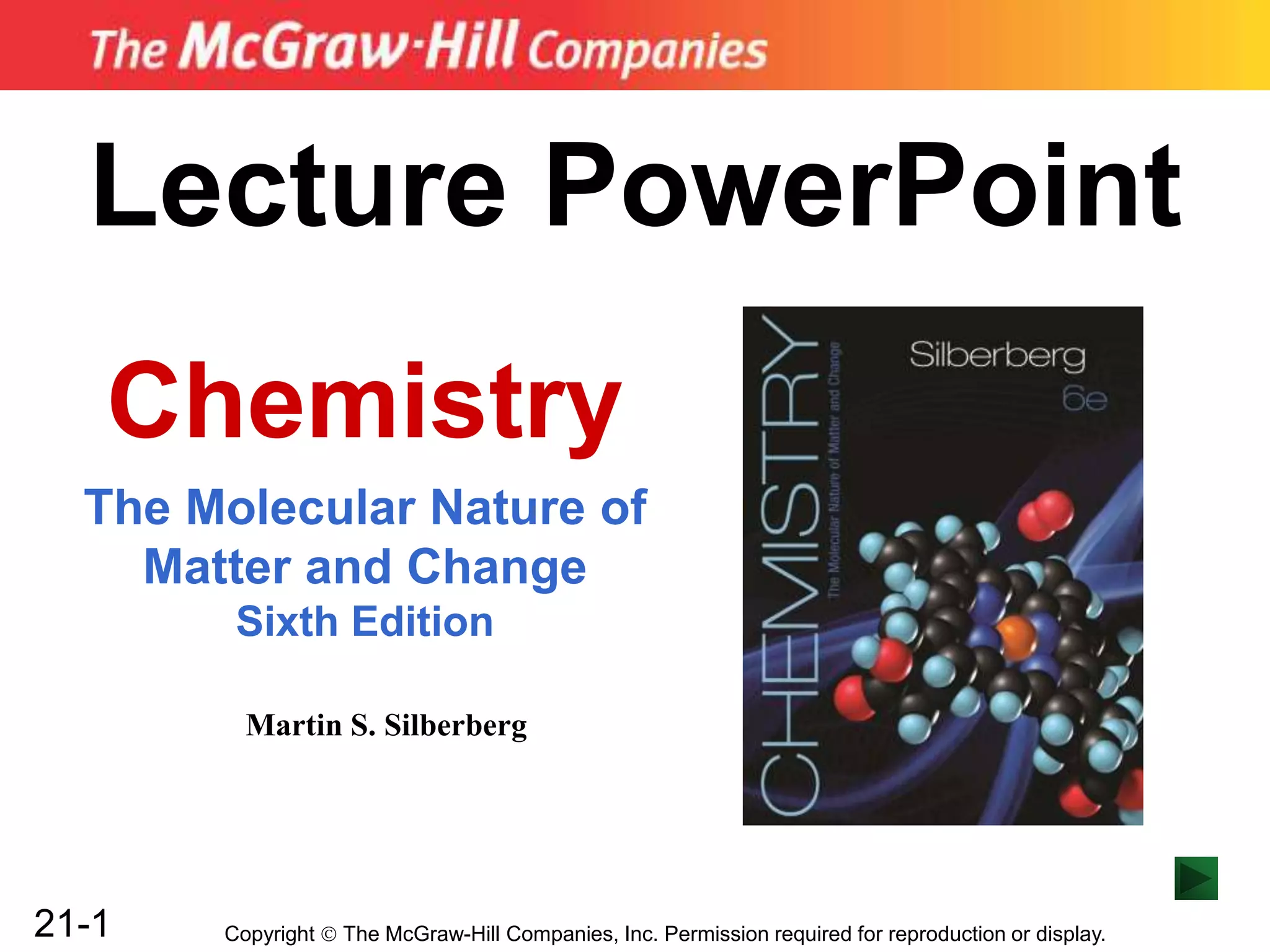
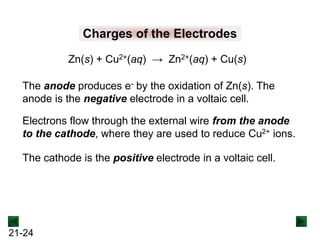
Spontaneous Redox Reactions
A strip of zinc metal in a solution of Cu2+ ions will react
spontaneously:
Cu2+(aq) + 2e- → Cu(s) [reduction]
Zn(s) → Zn2+(aq) + 2e- [oxidation]
Cu2+(aq) + Zn(s) → Zn2+(aq) + Cu(s)
Zn is oxidized, and loses electrons to Cu2+.
Although e- are being transferred, electrical energy is not
generated because the reacting substances are in the
same container.](https://image.slidesharecdn.com/ch21electrochem6efinal-210505161609/85/Ch21-electrochem-6e_final-18-320.jpg)
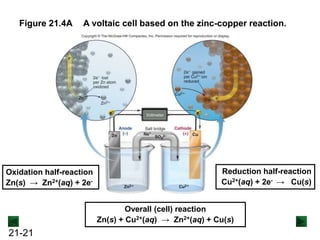
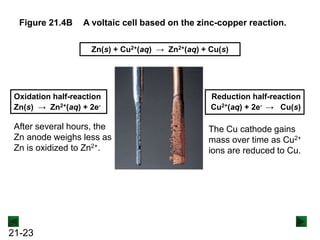
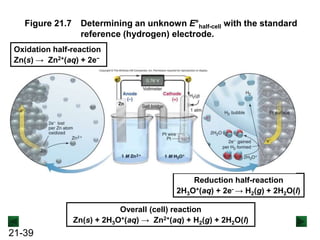
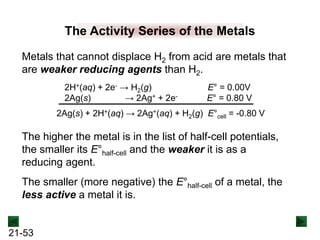
![21-22
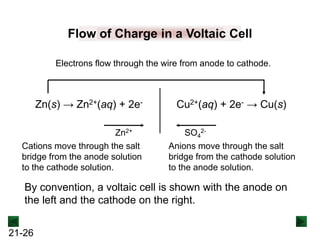
Operation of the Voltaic Cell
Oxidation (loss of e-) occurs at the anode, which is
therefore the source of e-.
Zn(s) → Zn2+(aq) + 2e-
Over time, the Zn(s) anode decreases in mass and the
[Zn2+] in the electrolyte solution increases.
Reduction (gain of e-) occurs at the cathode, where the e-
are used up.
Cu2+(aq) + 2e- → Cu(s)
Over time, the [Cu2+] in this half-cell decreases and the
mass of the Cu(s) cathode increases.](https://image.slidesharecdn.com/ch21electrochem6efinal-210505161609/85/Ch21-electrochem-6e_final-22-320.jpg)


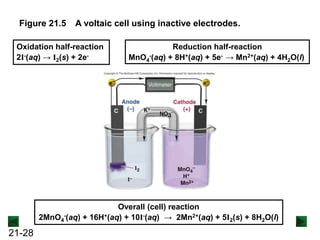
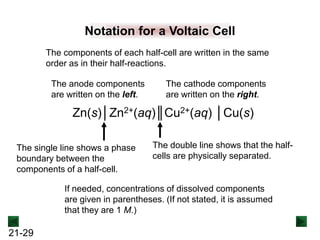

![21-32
SOLUTION:
Sample Problem 21.2
Ag+(aq) + e- → Ag(s) [reduction; cathode]
Cr(s) → Cr3+(aq) + 3e- [oxidation; anode]
3Ag+ + Cr(s) → 3Ag(s) + Cr3+(aq)
The half-reactions are:
The balanced overall equation is:
The cell notation is given by:
Cr(s)│Cr3+(aq)║Ag+(aq)│Ag(s)
The cell diagram shows the anode on
the left and the cathode on the right.](https://image.slidesharecdn.com/ch21electrochem6efinal-210505161609/85/Ch21-electrochem-6e_final-32-320.jpg)

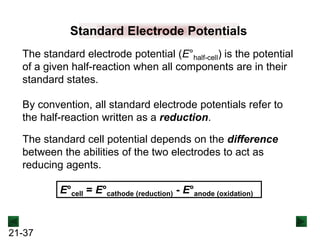

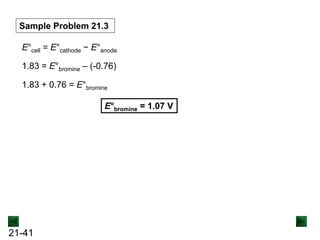
![21-40
Sample Problem 21.3 Calculating an Unknown E°half-cell from E°cell
PROBLEM: A voltaic cell houses the reaction between aqueous bromine
and zinc metal:
Br2(aq) + Zn(s) → Zn2+(aq) + 2Br-(aq) E°cell = 1.83 V.
Calculate E°bromine, given that E°zInc = -0.76 V
PLAN: E°cell is positive, so the reaction is spontaneous as
written. By dividing the reaction into half-reactions, we
see that Br2 is reduced and Zn is oxidized; thus, the zinc
half-cell contains the anode. We can use the equation for
E°cell to calculate E°bromine.
SOLUTION:
Br2(aq) + 2e- → 2Br-(aq) [reduction; cathode]
Zn(s) → Zn2+(aq) + 2e- [oxidation; anode] E°zinc = -0.76 V](https://image.slidesharecdn.com/ch21electrochem6efinal-210505161609/85/Ch21-electrochem-6e_final-40-320.jpg)

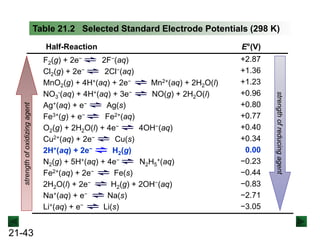

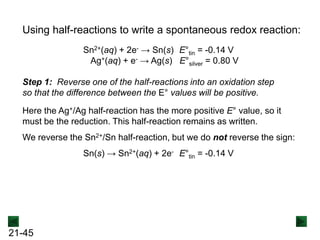
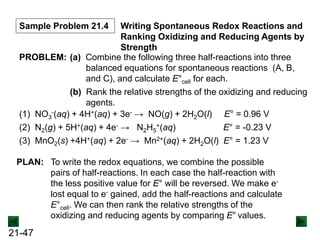
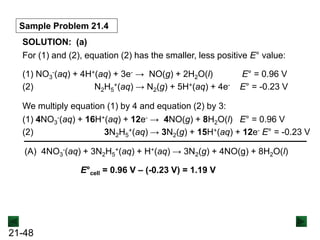

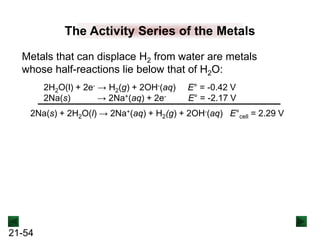
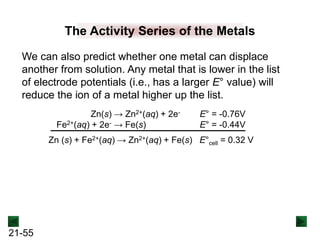
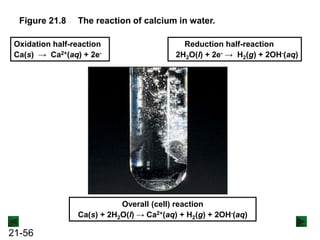
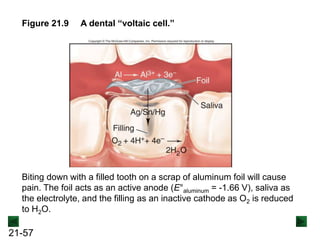
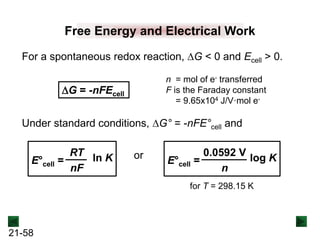
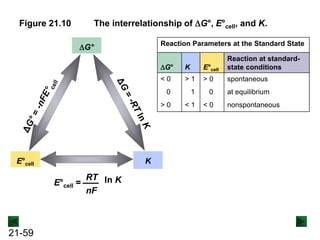
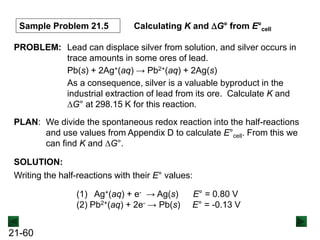
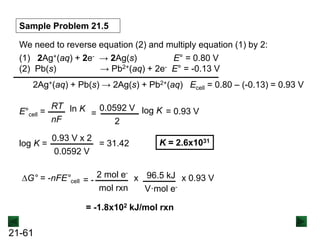
![21-62
Cell Potential and Concentration
• When Q < 1, [reactant] > [product], ln Q < 0, so Ecell > E°cell
• When Q = 1, [reactant] = [product], ln Q = 0, so Ecell = E°cell
• When Q > 1, [reactant] < [product], ln Q > 0, so Ecell < E°cell
Ecell = E°cell - log Q
0.0592 V
n
We can simplify the equation as before for T = 298.15 K:
Nernst Equation Ecell = E°cell - ln Q
RT
nF](https://image.slidesharecdn.com/ch21electrochem6efinal-210505161609/85/Ch21-electrochem-6e_final-62-320.jpg)
![21-63
Sample Problem 21.6 Using the Nernst Equation to Calculate Ecell
PROBLEM: In a test of a new reference electrode, a chemist constructs
a voltaic cell consisting of a Zn/Zn2+ half-cell and an H2/H+
half-cell under the following conditions:
[Zn2+] = 0.010 M [H+] = 2.5 M P = 0.30 atm
H2
Calculate Ecell at 298 K.
PLAN: To apply the Nernst equation and determine Ecell, we must
know E°cell and Q. We write the equation for the spontaneous
reaction and calculate E°cell from standard electrode
potentials. We must convert the given pressure to molarity in
order to have consistent units.
SOLUTION:
(1) 2H+(aq) + 2e- → H2(g) E° = 0.00 V
(2) Zn(s) → Zn2+(aq) + 2e- E° = -0.76 V
2H+(aq) + Zn(s) → H2(g) + Zn2+(aq) E°cell = 0.00 – (-0.76) = 0.76 V](https://image.slidesharecdn.com/ch21electrochem6efinal-210505161609/85/Ch21-electrochem-6e_final-63-320.jpg)
![21-64
Sample Problem 21.6
Converting pressure to molarity:
n
V
=
P
RT
=
0.30 atm
atm·L
mol·K
0.0821 x 298.15 K
= 1.2x10-2 M
[H2][Zn2+]
[H+]2
Q =
0.012 x 0.010
(2.5)2
= = 1.9x10-5
Solving for Ecell at 25°C (298.15 K), with n = 2:
Ecell = E°cell - log Q
0.0592 V
n
= 1.10 V -
0.0592 V
2
log(1.9x10-5) = 0.76 – (-0.14 V) = 0.90 V](https://image.slidesharecdn.com/ch21electrochem6efinal-210505161609/85/Ch21-electrochem-6e_final-64-320.jpg)
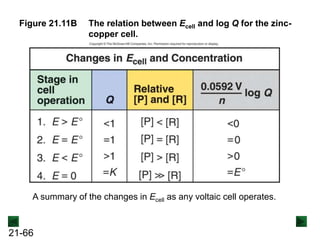
![21-65
Figure 21.11A The relation between Ecell and log Q for the zinc-
copper cell.
If the reaction starts with [Zn2+] < [Cu2+] (Q < 1), Ecell is higher than the
standard cell potential.
As the reaction proceeds, [Zn2+] decreases and [Cu2+] increases, so
Ecell drops. Eventually the system reaches equilibrium and the cell can
no longer do work.](https://image.slidesharecdn.com/ch21electrochem6efinal-210505161609/85/Ch21-electrochem-6e_final-65-320.jpg)
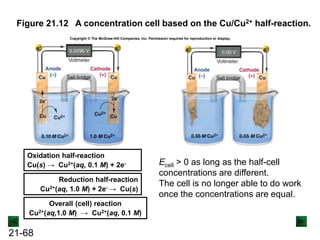
![21-67
Concentration Cells
A concentration cell exploits the effect of concentration
changes on cell potential.
The cell has the same half-reaction in both cell
compartments, but with different concentrations of
electrolyte:
Cu(s) → Cu2+(aq; 0.10 M) + 2e- [anode; oxidation]
Cu2+(aq; 1.0 M) → Cu(s) [cathode; reduction]
Cu2+(aq; 1.0 M) → Cu2+(aq; 0.10 M)
As long as the concentrations of the solutions are
different, the cell potential is > 0 and the cell can do work.](https://image.slidesharecdn.com/ch21electrochem6efinal-210505161609/85/Ch21-electrochem-6e_final-67-320.jpg)
![21-69
Sample Problem 21.7 Calculating the Potential of a Concentration
Cell
PROBLEM: A concentration cell consists of two Ag/Ag+ half-cells. In
half-cell A, the electrolyte is 0.0100 M AgNO3; in half-cell
B, it is 4.0x10-4 M AgNO3. What is the cell potential at
298.15 K?
PLAN: The standard half-cell potentials are identical, so E°cell is
zero, and we find Ecell from the Nernst equation. Half-cell A
has a higher [Ag+], so Ag+ ions are reduced and plate out on
electrode A, which is therefore the cathode. In half-cell B, Ag
atoms of the electrode are oxidized and Ag+ ions enter the
solution. Electrode B is thus the anode. As for all voltaic cells,
the cathode is positive and the anode is negative.](https://image.slidesharecdn.com/ch21electrochem6efinal-210505161609/85/Ch21-electrochem-6e_final-69-320.jpg)
![21-70
Sample Problem 21.7
= 0.0828 V
Ecell = E°cell - log
0.0592 V
1
[Ag+]dil
[Ag+]conc
= 0.0 V - 0.0592 log
4.0x10-4
0.010
SOLUTION: The [Ag+] decreases in half-cell A and increases in half-
cell B, so the spontaneous reaction is:
Ag+(aq; 0.010 M) [half-cell A] → Ag+(aq; 4.0x10-4 M) [half-cell B]](https://image.slidesharecdn.com/ch21electrochem6efinal-210505161609/85/Ch21-electrochem-6e_final-70-320.jpg)
![21-71
Figure 21.13 Laboratory measurement of pH.
The operation of a pH meter illustrates an important application of
concentration cells. The glass electrode monitors the [H+] of the
solution relative to its own fixed internal [H+].
An older style of pH meter
includes two electrodes.
Modern pH meters use a
combination electrode.](https://image.slidesharecdn.com/ch21electrochem6efinal-210505161609/85/Ch21-electrochem-6e_final-71-320.jpg)
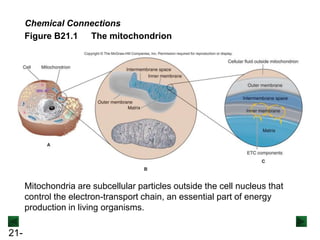
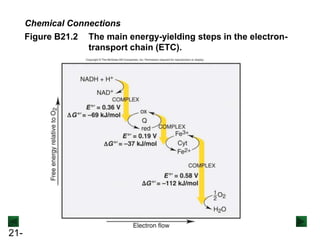
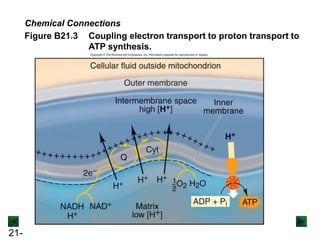
![21-73
Figure 21.14 Minimocroanalysis.
A microelectrode records electrical impulses of a single neuron in a
monkey’s visual cortex. The electrical potential of a nerve cell is due to
the difference in concentration of [Na+] and [K+] ions inside and outside
the cell.](https://image.slidesharecdn.com/ch21electrochem6efinal-210505161609/85/Ch21-electrochem-6e_final-73-320.jpg)

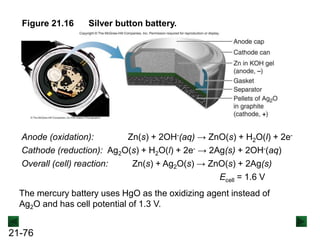
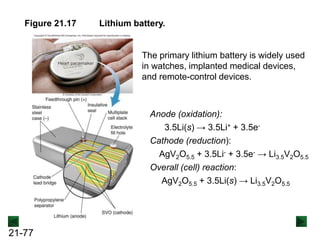
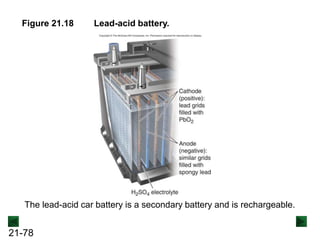
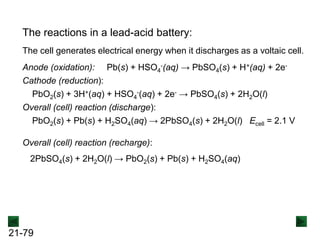

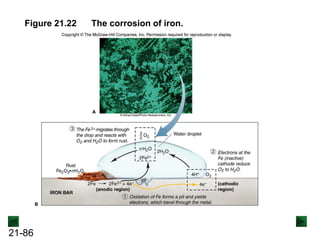
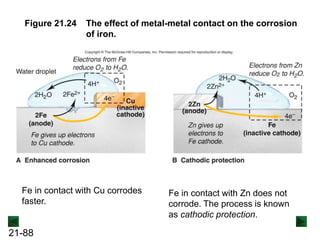
![21-85
The Rusting of Iron
Fe(s) → Fe2+(aq) + 2e- [anodic region; oxidation]
O2(g) + 4H+(aq) + 4e- → 2H2O(l) [cathodic region; reduction]
The loss of iron:
2Fe(s) + O2(g) + 4H+(aq) → 2Fe2+(aq) + 2H2O(l) [overall]
The rusting process:
2Fe2+(aq) + ½O2(g) + (2 + n)H2O(l) → Fe2O3·nH2O(s) + 4H+(aq)
Overall reaction:
H+ ions are consumed in the first step, so lowering the pH increases the
overall rate of the process. H+ ions act as a catalyst, since they are
regenerated in the second part of the process.](https://image.slidesharecdn.com/ch21electrochem6efinal-210505161609/85/Ch21-electrochem-6e_final-85-320.jpg)


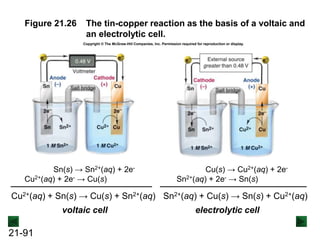
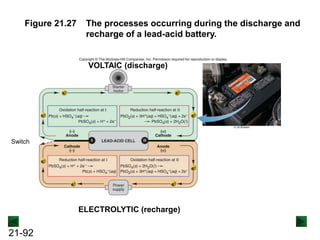
![21-90
Electrolytic Cells
An electrolytic cell uses electrical energy from an
external source to drive a nonspontaneous redox
reaction.
Cu(s) → Cu2+(aq) + 2e- [anode; oxidation]
Sn2+(aq) + 2e- → Sn(s) [cathode; reduction]
Cu(s) + Sn2+(aq) → Cu2+(aq) + Sn(s) E°cell = -0.48 V and ΔG° = 93 kJ
As with a voltaic cell, oxidation occurs at the anode and
reduction takes place at the cathode.
An external source supplies the cathode with electrons,
which is negative, and removes then from the anode,
which is positive. Electrons flow from cathode to anode.](https://image.slidesharecdn.com/ch21electrochem6efinal-210505161609/85/Ch21-electrochem-6e_final-90-320.jpg)


![21-96
Sample Problem 21.8
SOLUTION:
Possible oxidizing agents: Na+, Mg2+
Possible reducing agents: Br-, Cl-
Mg is to the right of Na in Period 3. IE increases from left to right across
the period, so Mg has the higher IE and gives up its electrons less
easily. The Mg2+ ion has a greater attraction for e- than the Na+ ion.
Br is below Cl in Group 7A. EN decreases down the group, so Br
accepts e- less readily than Cl. The Br- ion will lose its e- more easily, so
it is more easily oxidized.
Mg2+(l) + 2e- → Mg(l) [cathode; reduction]
2Br-(l) → Br2(g) + 2e- [anode; oxidation]
The overall cell reaction is: Mg2+(l) + 2Br-(l) → Mg(l) + Br2(g)](https://image.slidesharecdn.com/ch21electrochem6efinal-210505161609/85/Ch21-electrochem-6e_final-96-320.jpg)