Embed presentation
Download as PDF, PPTX

![Redox
If given an unfamiliar species, e.g. [Co(NH3)6]2+,
how do I know what can it oxidise/ reduce to?
Look for the species in data booklet.](https://image.slidesharecdn.com/redox-110927191439-phpapp02/85/Redox-2-320.jpg)
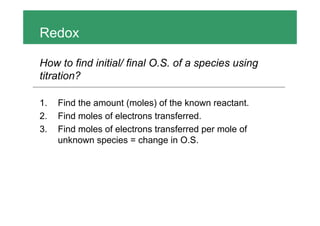
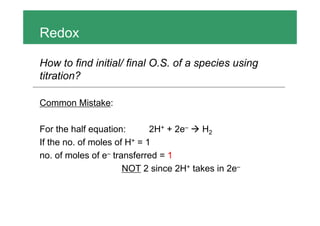
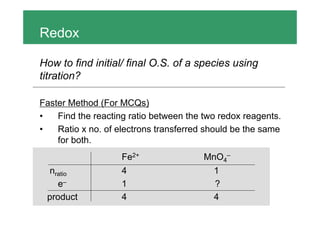
To find the initial and final oxidation states of a species using a titration: 1. Find the moles of the known reactant and electrons transferred 2. Calculate the moles of electrons transferred per mole of the unknown species to get the change in oxidation state 3. A common mistake is thinking the number of moles of electrons transferred is equal to the coefficient in the half-reaction, when it is actually the number of electrons transferred per mole of reactants.

![Redox
If given an unfamiliar species, e.g. [Co(NH3)6]2+,
how do I know what can it oxidise/ reduce to?
Look for the species in data booklet.](https://image.slidesharecdn.com/redox-110927191439-phpapp02/85/Redox-2-320.jpg)


