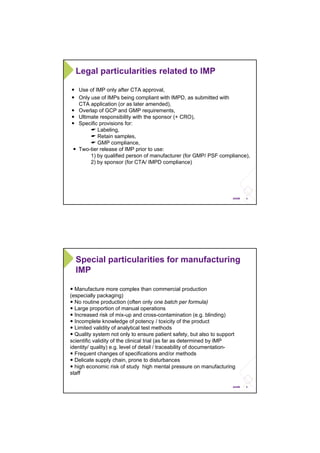
This document outlines the legal framework and GMP requirements for manufacturing investigational medicinal products (IMPs) used in clinical trials. It discusses what constitutes an IMP and the unique challenges of IMP manufacturing, including a lack of routine production and increased risk of mix-ups. The key chapters of Annex 13 of the EU GMP guide are summarized, focusing on quality management, documentation, production, quality control, and batch release as they apply to IMPs. Inspections of IMP manufacturing sites should ensure compliance with GMP requirements and identification of specific standard operating procedures for IMPs.






![14ANSM
Contents of GMP inspection at IMP manufacturing
site
Regarding the premises and equipment:
Design suitable to prevent cross-contamination by potentially toxic
or sensitising materials:
Cleanability,
Containment,
Staff / materials flow,
Warehouse:
sufficient space, adequate segregation,
Qualified freezers, refrigerators,
Computerised systems validated e.g. label text databases, label
printers, random list generation, etc.
15ANSM
Regarding the documentation:
PSF: complete [next slide], up-to-date, compliant with IMPD,
Specifications & instructions (manufacturing, packaging, shipment /
distribution etc.) up-to-date, compliant with PSF,
Manufacturing order: detailed (<-> ref. to PSF), authorised,
Changes: rationales recorded, consequences investigated,
Records (manufacturing, packaging, testing, shipping):
sufficiently detailed (e.g. reconciliation of amounts),
changes / deviations logged.
Contents of GMP inspection at IMP manufacturing
site](https://image.slidesharecdn.com/eipg-bipa-11april2014-jacquesmorenas-150218173249-conversion-gate02/85/Implementation-of-Annex-13-of-the-EU-GMP-Guide-8-320.jpg)




