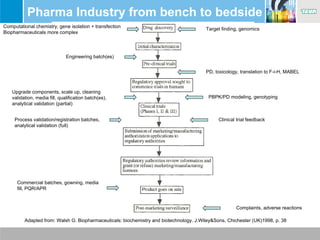
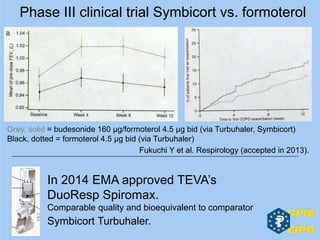
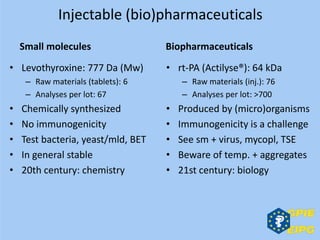
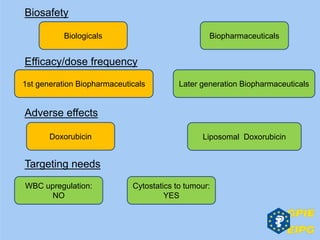
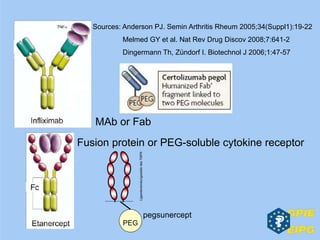
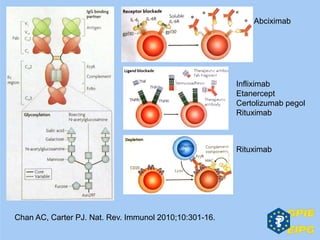
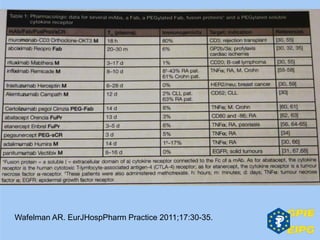
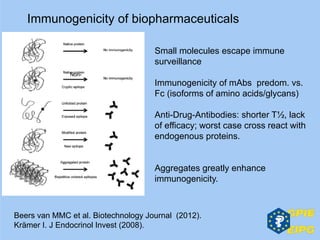
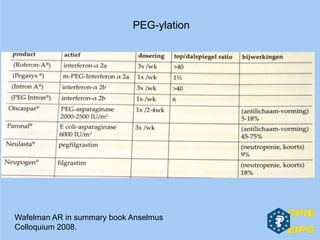
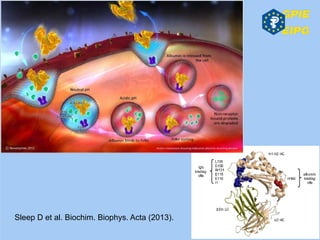
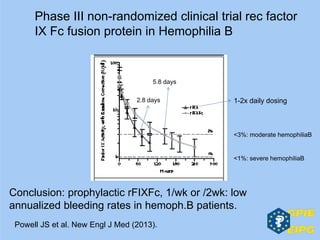
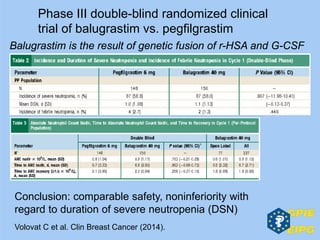
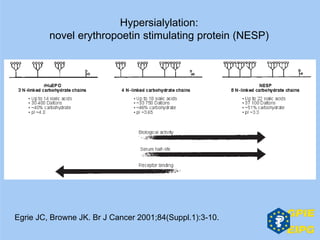
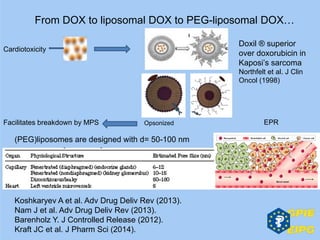
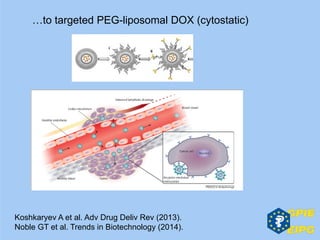
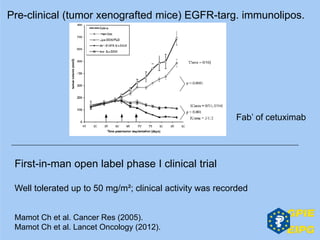
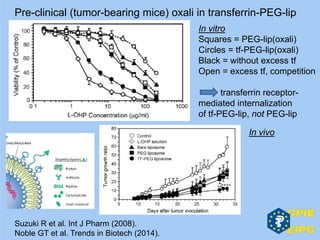
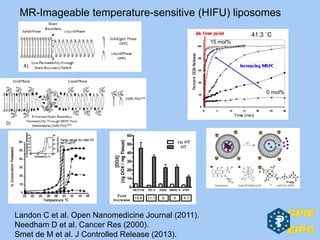
The document presents insights from the EPSA Autumn Symposium 2014 on the development of biopharmaceuticals, highlighting key aspects such as formulation considerations, biosafety, and immunogenicity challenges. It discusses the evolution from small molecules to complex proteins, emphasizing clinical trial data and the significance of proper formulation and delivery methods. The content also covers recent advancements in drug development, including liposomal formulations and pegylation techniques.