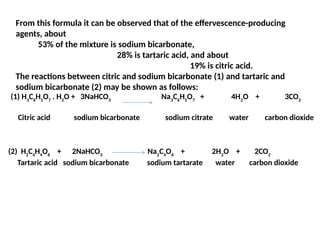
Effervescent granules are mixtures containing medicinal agents and a combination of sodium bicarbonate, citric acid, and tartaric acid, which produce carbon dioxide when dissolved in water, resulting in effervescence. They provide benefits such as improved taste acceptance and faster drug absorption and can be prepared through wet, dry, or fusion methods, adhering to specific compositions and storage guidelines. The document details the preparation methods, advantages, and chemical reactions involved in producing effervescent salts, emphasizing the importance of certain ratios of ingredients for efficacy and safety.