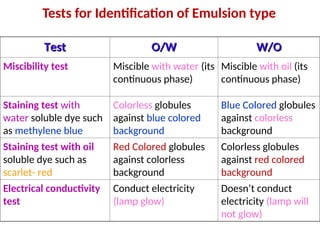
An emulsion is a dispersion of liquid globules in a non-miscible medium, and requires an emulsifying agent to maintain stability. There are two main types of emulsions: oil in water (o/w) and water in oil (w/o), differentiated through miscibility, staining, and conductivity tests. Emulsions serve important pharmaceutical and therapeutic purposes, enhancing drug administration and absorption while requiring careful formulation to ensure stability and prevent separation.













![Pharmaceutical application of microemulsion
Hydrophilic surfactants may be used to produce "transparent" o/w
emulsions of many oils, including flavour oils and vitamin oils such as A,
D, and E.
Surfactants in the HLB range of 15 to 18 have been used most extensively
in the preparation of such emulsions. These emulsions are dispersions of
oil, not true solutions; however, because of the appearance of the
product, the surfactant is common] said to "solubilize" the oil.
Surfactants common used in the preparation of such oral liquid
formulations are polysorpate 60 and polysorpate 80.
The advantages cited for the use of microemulsions in drug delivery are:
more rapid and efficient oral absorption of drugs than through solid
dosage forms; enhanced transdermal drug delivery through increased
drug diffusion into the skin;
O/W microemulsions are being formulated as aqueous vehicles for oil
soluble drugs to be administered by the percutaneous, oral or parenteral
route.](https://image.slidesharecdn.com/emulsioniii-241202094320-d9ab8165/85/emulsion_iii-ppt-a-16-320.jpg)










