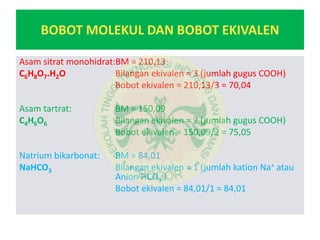
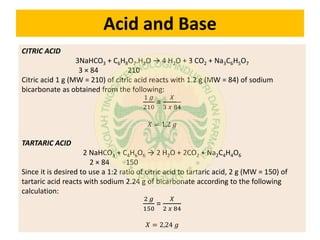
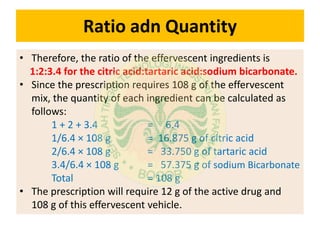
This document discusses the calculation of an effervescent formula for vitamin C tablets. It begins by providing the formula for a 1.5g effervescent tablet, which includes 500mg of vitamin C. It then calculates the formula based on the tablet weight, dividing it into inner and outer phases. The weights of the acid and base components are calculated based on their molecular weights and equivalents in the reaction equations provided. Ratios of citric acid, tartaric acid and sodium bicarbonate are determined for an effervescent granule example based on these calculations.