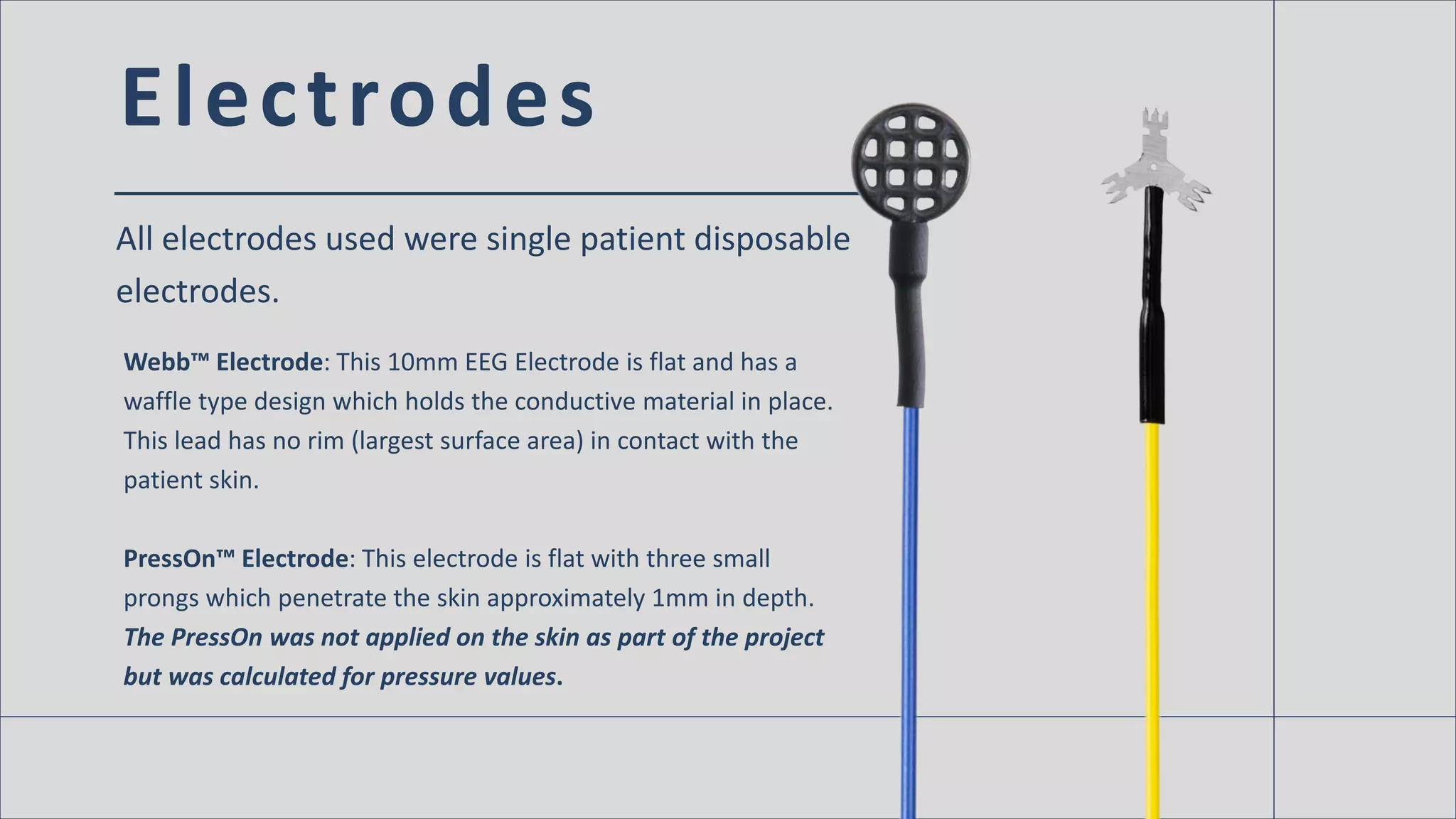
The document discusses the impact of EEG electrode design on skin safety and health, highlighting that traditional deep cup electrodes can increase the risk of skin breakdown due to their smaller surface area compared to slim cup and flat designs. Results indicate that the shape of electrodes affects the pressure applied to the skin, with smaller surface areas correlating with higher skin pressure and potential issues. The findings were presented at the 2016 Neurodiagnostic Society annual meeting, emphasizing the need for consideration of electrode design in patient care.