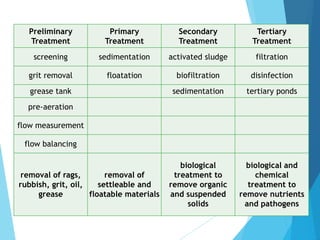
The document discusses wastewater management and treatment. It covers the definition of wastewater, sources of wastewater like households and industries, and effects of untreated wastewater like water pollution and health impacts. It then describes the wastewater treatment process which includes physical, chemical, and biological steps. Primary treatment involves screening and grit removal while secondary treatment uses biological processes like activated sludge. The document also discusses Malaysia's laws and policies around wastewater treatment and standards for treated effluent quality.