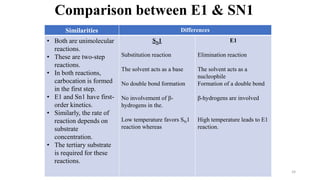
Elimination reactions involve the removal of two substituents from adjacent carbon atoms to form a double or triple bond. The document discusses E1 elimination reactions, which proceed through a two-step unimolecular mechanism. In the first step, a carbocation intermediate is formed. In the second step, a proton is removed from the carbocation rapidly to form the alkene product. E1 reactions favor substrates that form stable carbocations and are accelerated by polar protic solvents, weak bases, and high temperatures. The rate depends on the concentration of the substrate and the reaction follows first-order kinetics.





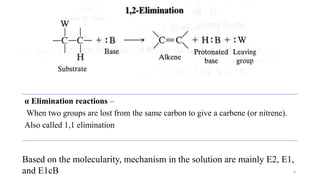

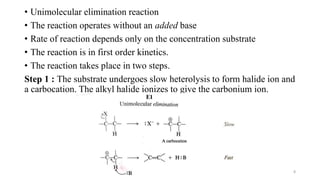

![• Here, the breaking of C-X bond is complete before any reaction occurs
with the base to lose H and before C=C is formed.
• Only alkyl halide is involved in the rate determining step
• So, the rate of reaction can be expressed as:
Rate ∝ [ substrate concentration]
Rate = k[alkyl halide]
• In pure E1 reaction, the product should be completely non-
stereospecific. This is because bond rotation is possible in the
carbocation before deprotonation.
10](https://image.slidesharecdn.com/eliminationreactions-240204133432-5c4a8680/85/Elimination-reactions-E1-Elimination-reaction-10-320.jpg)