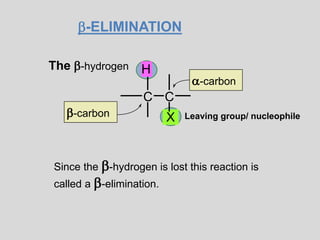
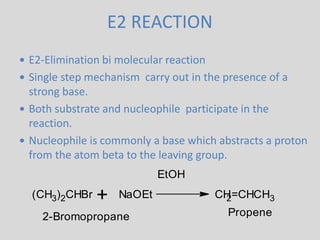
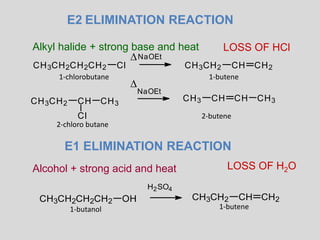
The document summarizes elimination reactions, which involve removing two substituents from a molecule in the presence of a base. It describes the E1 and E2 mechanisms, noting that E1 is first order and involves a carbocation, while E2 is second order. E2 requires an anti-coplanar orientation of the leaving groups and occurs more readily with secondary and tertiary substrates. The orientation of elimination is also discussed based on Saytzeff's and Hofmann's rules. Stereochemistry preferences, reactivity factors, and conclusions about elimination versus substitution are provided.




![TYPES OF ELIMINATION REACTION
• Elimination : Both the groups are lost from the same
carbon atom.eg: Dichlorocarbene formation
HCCl3 Base [:CCl2]
• Elimination : Halogen is lost from carbon and
Hydrogen is lost from carbon.
eg: dehydrohalogenation of alkyl halides
• Elimination- Departing groups located at 1,3or more
remote sites, cyclic products are formed.
H-O-CH2CH2CH2CH2-Cl
O](https://image.slidesharecdn.com/eliminationsheeja-221031071559-c5afd69d/85/ELIMINATION-SHEEJA-pptx-5-320.jpg)




![Elimination unimolecular,E1
Two-step process – ionization (carbocation)
and deprotonation.
Take place in the presence of only weak base.
Accompanied by carbocation rearrangement
Rate is 1st order
Compete with SN1 [common carbocationic
intermediate]
No deuterium isotope effect.](https://image.slidesharecdn.com/eliminationsheeja-221031071559-c5afd69d/85/ELIMINATION-SHEEJA-pptx-13-320.jpg)

![[RX] constant, [B] increasing
Rate
rate = k1 [RX]
E1
rate = k2 [RX] [B] E2
BEHAVIOUR OF THE RATE WITH
INCREASING BASE CONCENTRATION
second order
first order
E1 dominates
at low base
concentration E2 dominates
at higher base
concentration](https://image.slidesharecdn.com/eliminationsheeja-221031071559-c5afd69d/85/ELIMINATION-SHEEJA-pptx-16-320.jpg)








![Deuterated norbornyl bromide [X-Br]
D
H
H
X
H
H
exo
endo
Rigid molecule
SYN - ELIMINATION
SYN ELIMINATION OCCURS BECAUSE
THERE ARE NO ANTI-COPLANAR -H](https://image.slidesharecdn.com/eliminationsheeja-221031071559-c5afd69d/85/ELIMINATION-SHEEJA-pptx-25-320.jpg)












