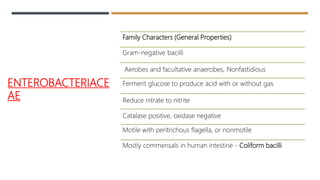
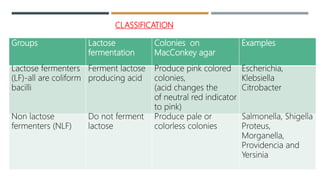
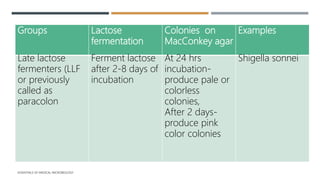
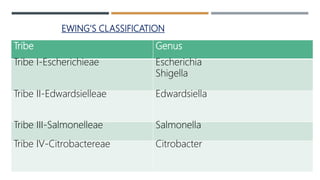
E. coli is a gram-negative, facultative anaerobic rod that is commonly found in the gut of humans and animals. It has various virulence factors like somatic (O), flagellar (H), capsular (K) antigens and fimbriae that aid in colonization and pathogenesis. Major diseases caused are urinary tract infections, diarrhea, and other infections. Diarrheagenic E. coli are classified into six types based on virulence mechanisms - EPEC, ETEC, EIEC, EHEC/VTEC, EAEC, DAEC. EHEC secretes Shiga toxin/verocytotoxin which can cause hemorrhagic colitis and HUS. U