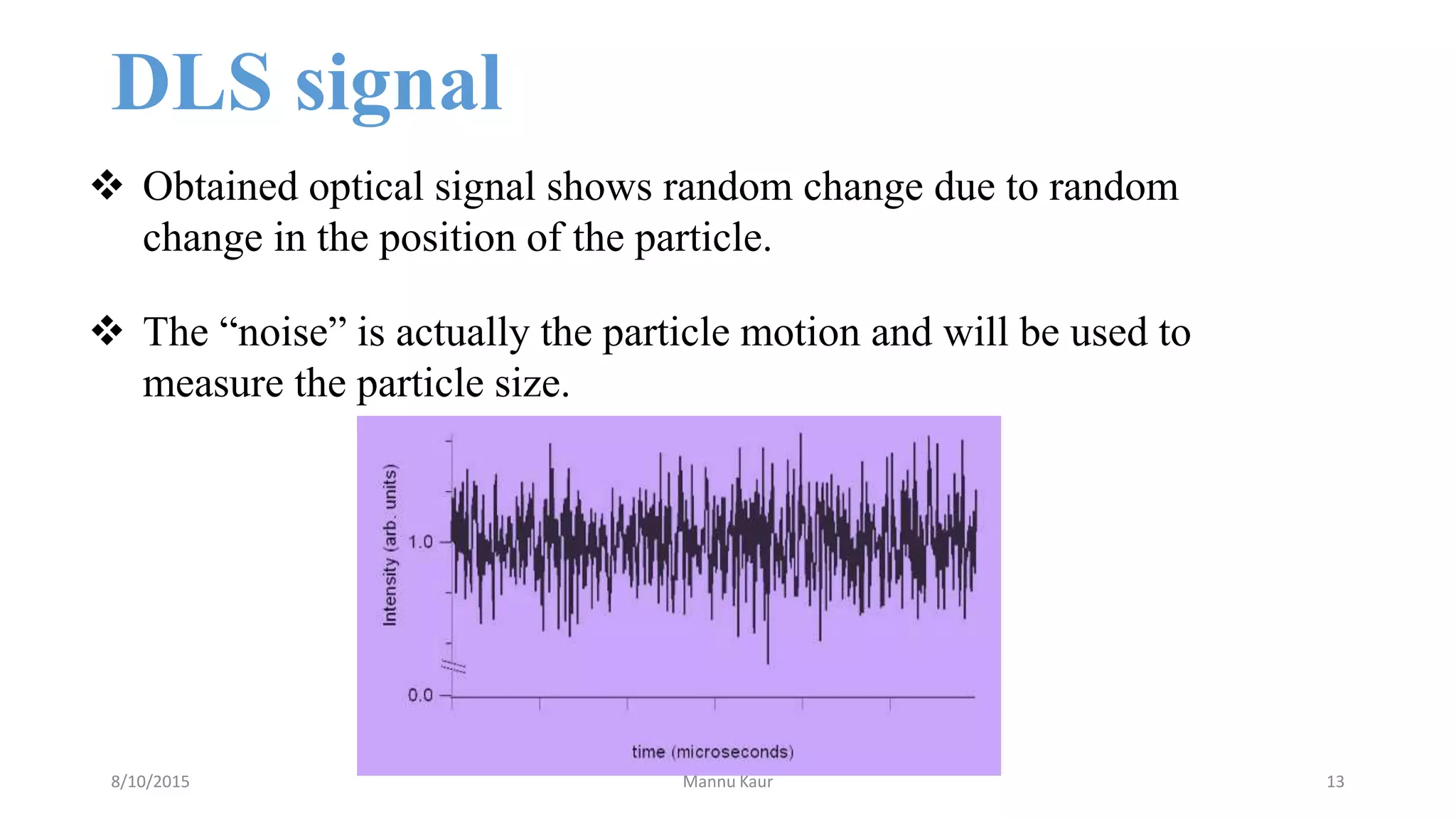
This document discusses dynamic light scattering (DLS) for determining particle size in colloidal systems. It explains that DLS measures the hydrodynamic diameter of particles by analyzing how light scatters off particles undergoing Brownian motion. The speed of this motion is related to particle size via the Stokes-Einstein equation. DLS has applications in characterization of proteins, polymers, micelles and nanoparticles. It also allows monitoring of particle aggregation over time.