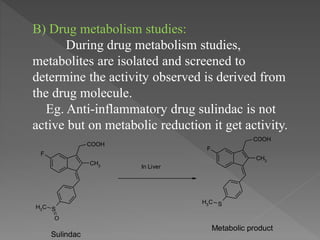
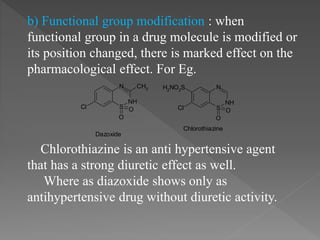
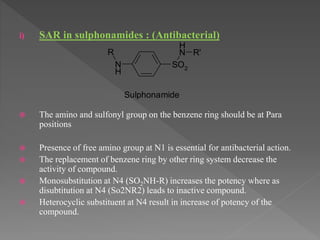
The document outlines the drug discovery and development process. It begins with discovery of a lead compound, which then undergoes optimization and preclinical testing. This involves modifying the compound's structure to improve efficacy and safety while reducing toxicity. Compounds then enter three phases of clinical trials in humans to test safety, efficacy, and proper dosing. The entire process from discovery to market approval takes an average of 12-15 years. Structure-activity relationships are also studied to understand how modifications impact a drug's effects.

![2
Drug Discovery Pathway
Efficacy
Toxicology
Safety
Preformulations
Stability Studies
Leads
Selection of
candidate drug
Preclinical Studies
Primary Screening
[Hits]
Discovery
&
Development](https://image.slidesharecdn.com/druganddyes-190504083122/85/Drug-Discovery-2-320.jpg)