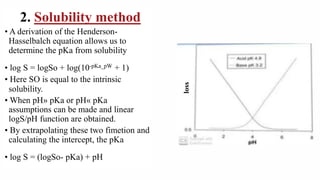
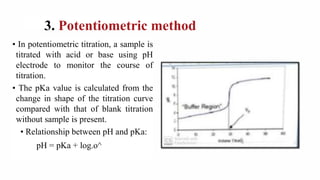
This document discusses dissociation constants and methods for their determination. It defines dissociation constant as the tendency of a substance in solution to reversibly dissociate into ions. Common methods for determining dissociation constants include conductivity, solubility, and potentiometric titration methods. The document also discusses the Henderson-Hasselbalch equation and how dissociation constants relate to acid strength and percent ionization in solutions.



![• Degree of ionization depends on pH
• Henderson-Hasselbalch equation:
• For basic compounds:
pH = pKa +
[Ionized]
un-ionized]
• For acidic compounds:
pH = pKa +
[un-ionized]
[ionizeds]](https://image.slidesharecdn.com/dissociationconstant-230625194039-57748d34/85/Dissociation-Constant-4-320.jpg)