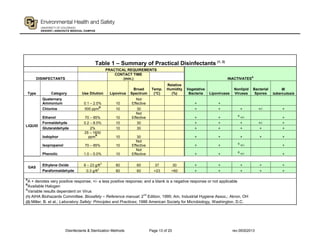
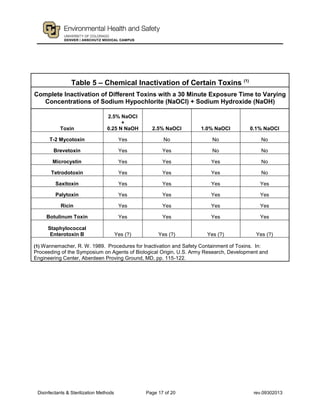
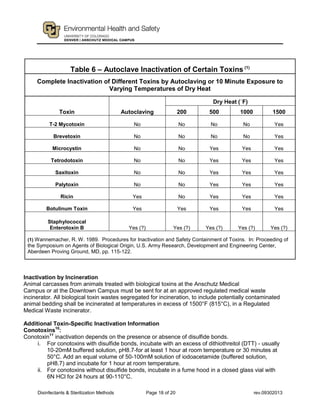
This document provides guidance on disinfectants and sterilization methods. It defines key terms and discusses various classes of chemical disinfectants like aldehydes, halogen compounds, quaternary ammonium compounds, phenolics, acids/alkalis, heavy metals and alcohols. It also covers sterilization methods such as steam autoclaving, dry heat, radiation and vapors/gases. Tables provide summaries of practical disinfectants and their characteristics, potential applications and examples of proprietary disinfectants. The document aims to assist in selecting appropriate disinfectants and sterilization methods.