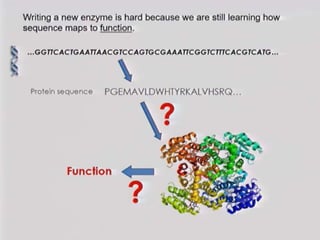
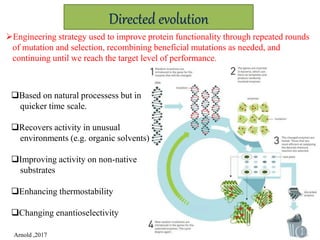
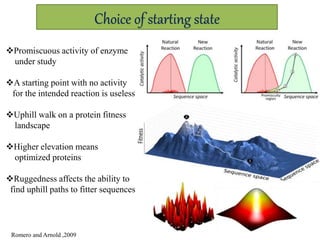
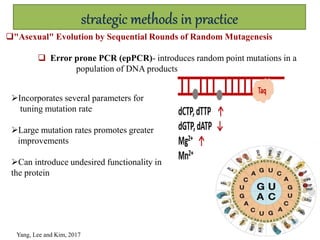
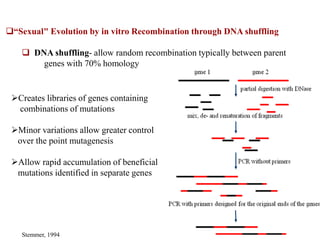
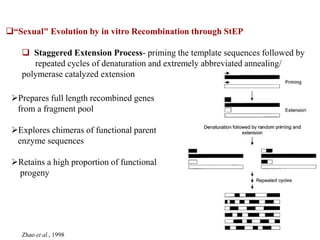
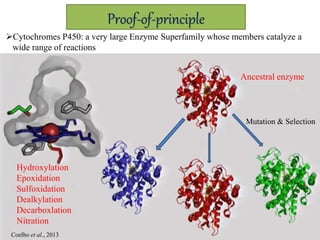
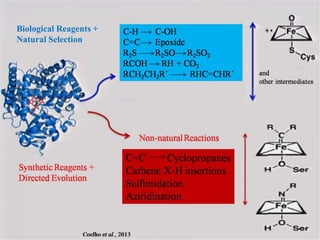
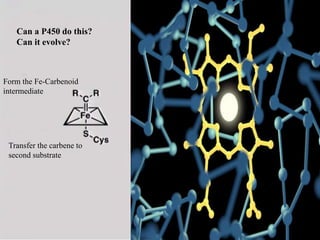
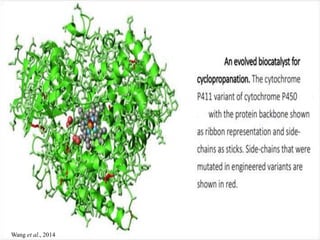
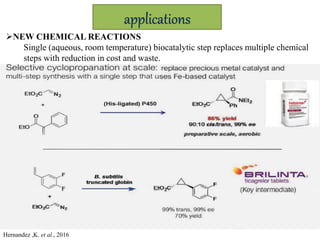
This document discusses directed evolution of enzymes. It describes how Frances Arnold developed directed evolution techniques including error-prone PCR, DNA shuffling, and StEP to improve enzyme functionality for desired traits like activity, stability, selectivity. Her techniques combine random mutagenesis and recombination with selection to evolve enzymes, allowing optimization and redesigning of enzyme function. Directed evolution has led to enzymes catalyzing new reactions and expanded our understanding of what reactions enzymes can catalyze.