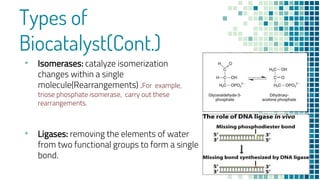
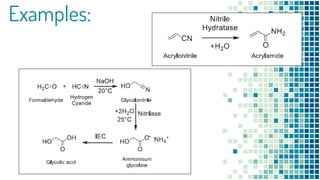
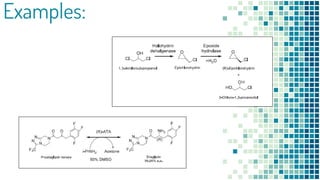
This document discusses biocatalysis and provides an overview of enzymes and their uses. It defines biocatalysts as substances like enzymes that catalyze chemical reactions in living organisms. The document outlines the sources and production of enzymes, the main types of biocatalysts including hydrolases and lyases, important human enzymes like amylase, and factors that affect enzymatic activity such as temperature, pH, and inhibitors. It also discusses applications of biocatalysis in areas like DNA replication, medical diagnostics and therapeutics, and industries including pharmaceutical, food, textiles, and more. In closing, it notes the possibilities and challenges of biocatalysts in industrial sectors.