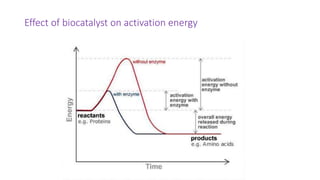
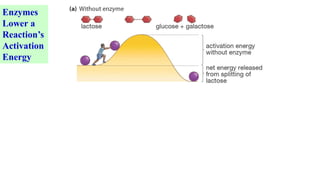
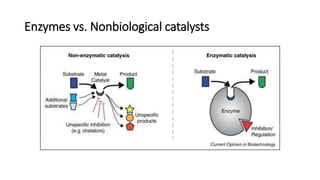
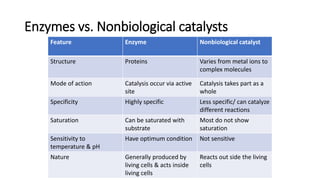
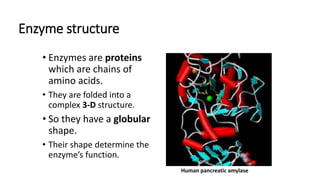
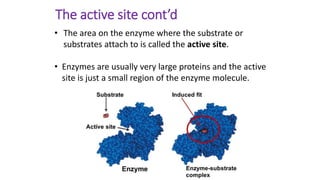
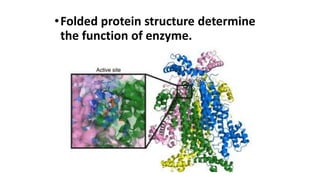
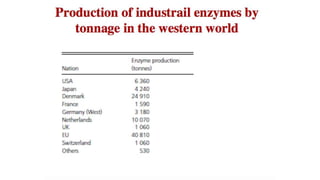
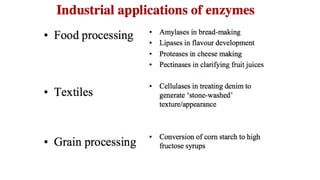
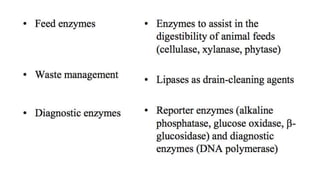
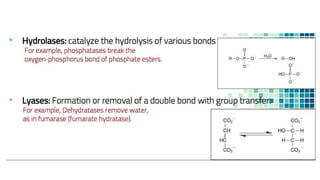
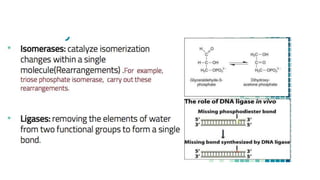
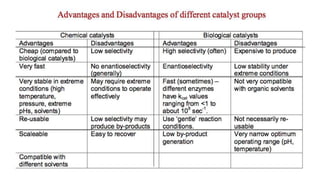
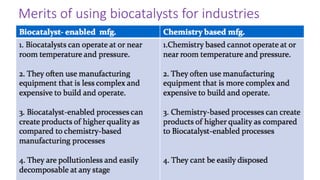
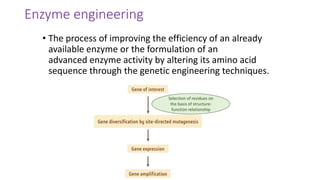
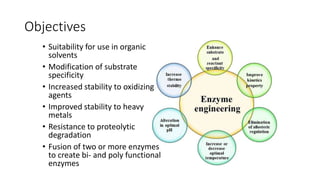
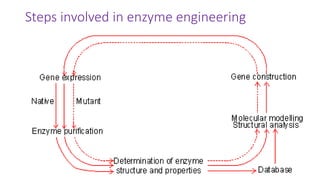
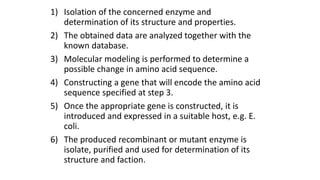
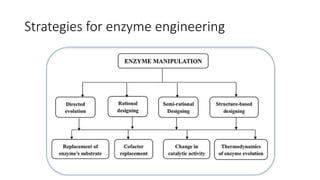
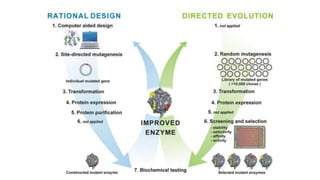
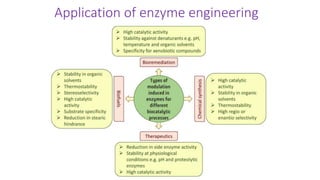
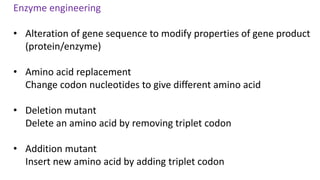
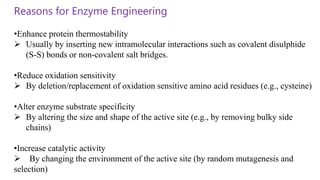
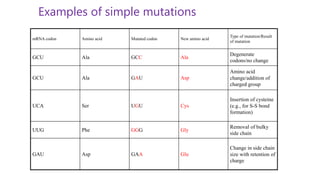
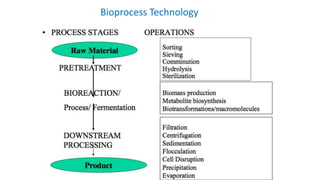
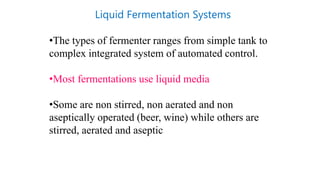
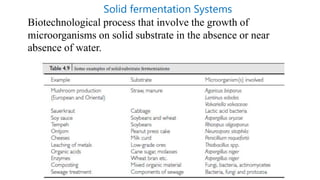
This document discusses biocatalysts and enzymes. It begins by listing five properties of useful industrial microbes, such as producing spores or being amenable to genetic manipulation. It then defines biocatalysts as enzymes or microbes that accelerate chemical reactions. Enzymes function as biocatalysts by lowering the activation energy of reactions. The document outlines the structure and function of enzymes, including their active sites, and compares enzymes to non-biological catalysts. It also discusses producing biocatalysts through fermentation and engineering enzymes to modify their properties.