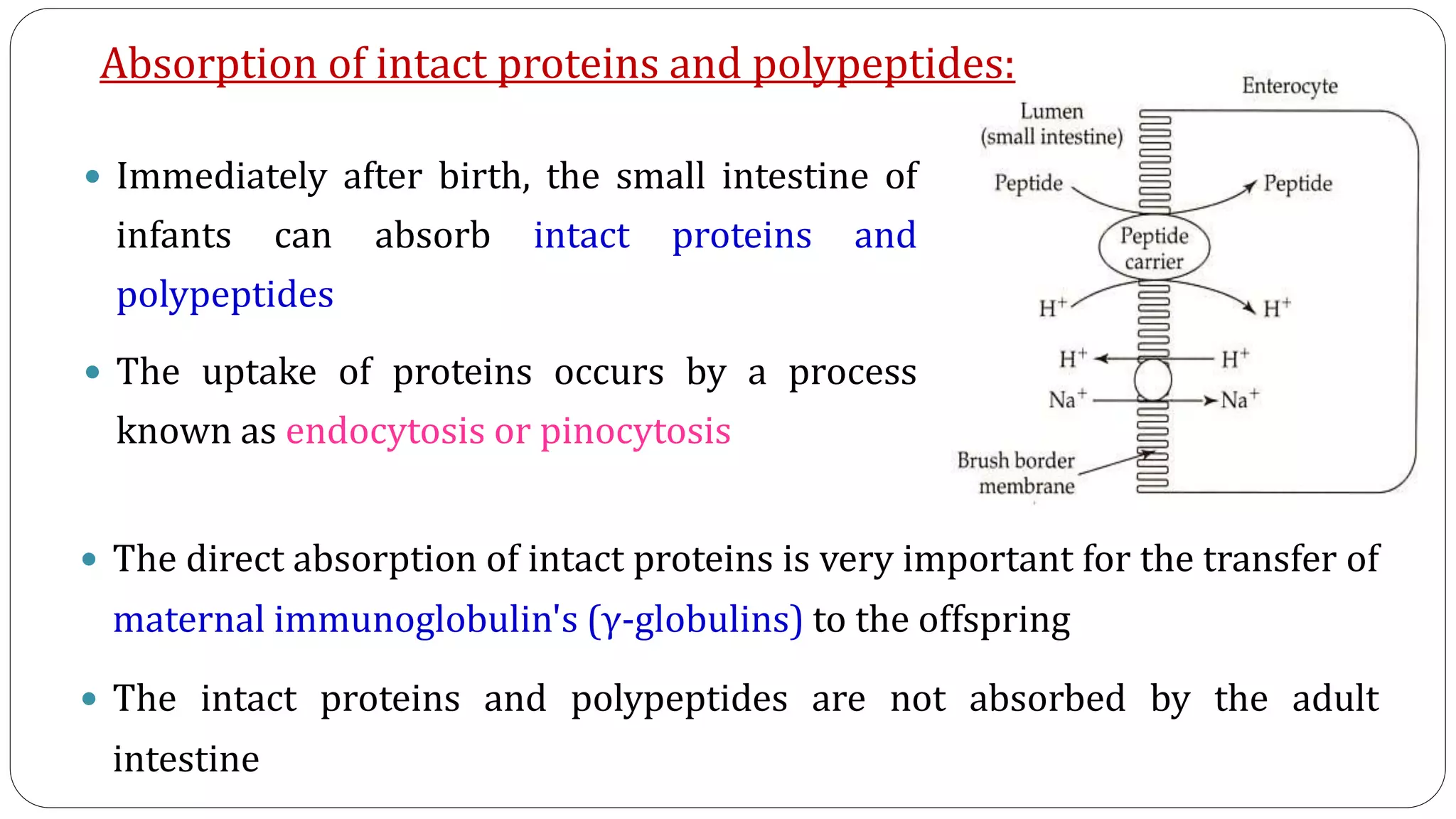
This document summarizes the digestion and absorption of proteins in the human body. It discusses that proteins are obtained endogenously from digestive enzymes and cells, as well as exogenously from dietary intake. The stomach contains hydrochloric acid and pepsin to denature and break down proteins. The pancreas secretes trypsinogen, chymotrypsinogen, and other zymogens which are activated and further digest proteins into peptides and amino acids in the small intestine. Aminopeptidases and dipeptidases on intestinal cells complete the digestion. Amino acids are then absorbed via active transport systems involving sodium and ATP. Deficiencies or defects in these digestive processes can impair protein digestion.