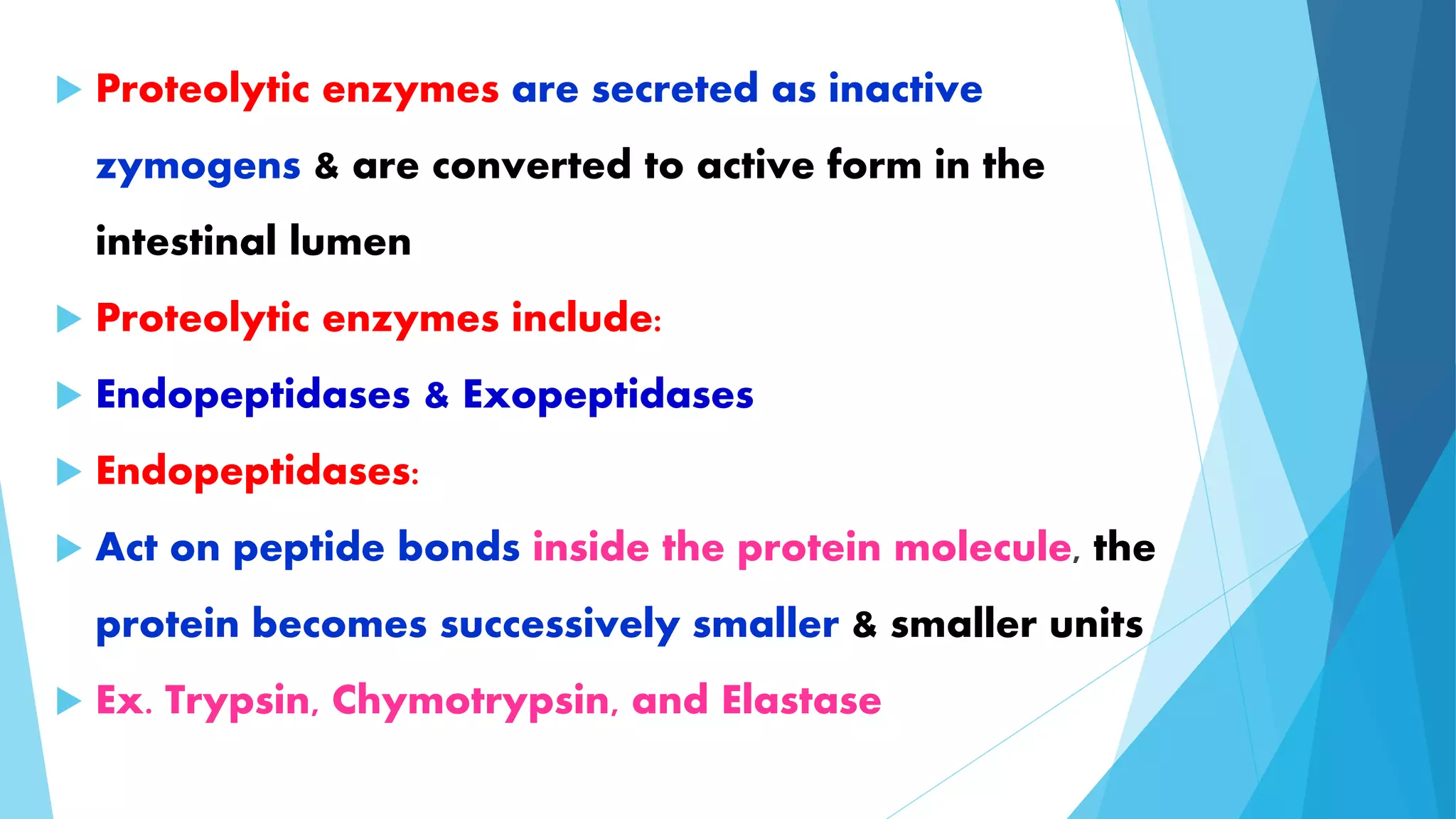
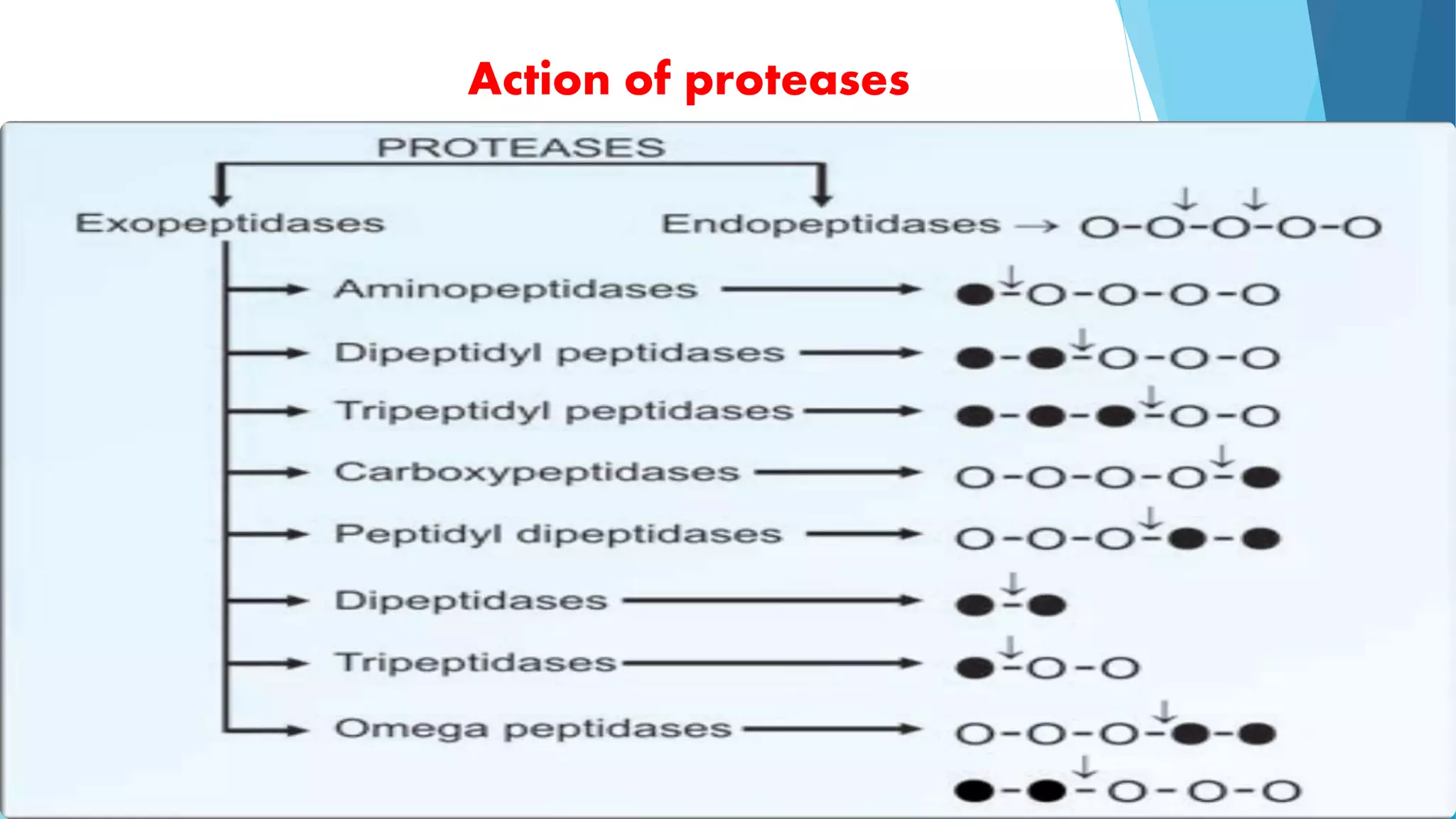
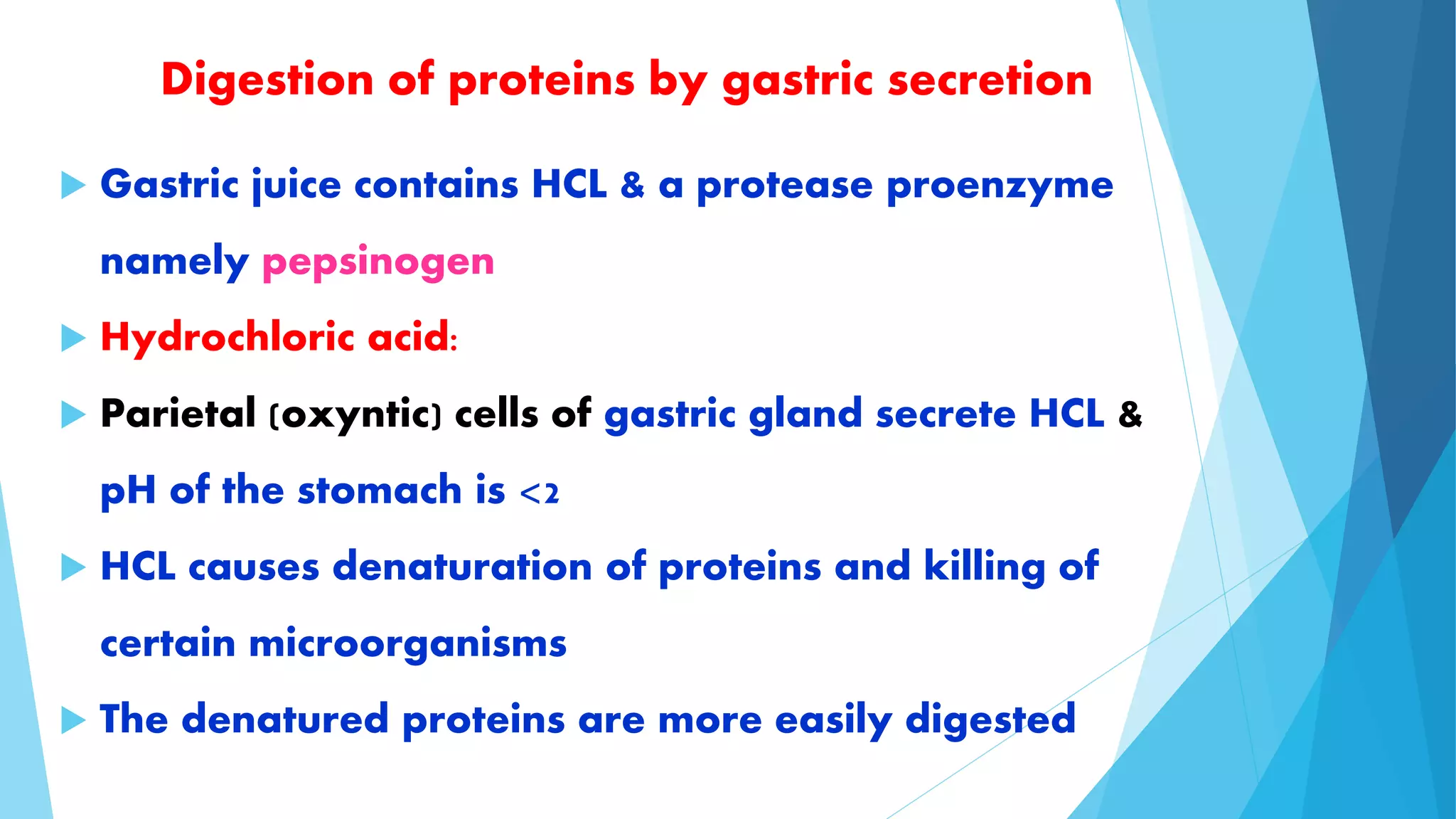
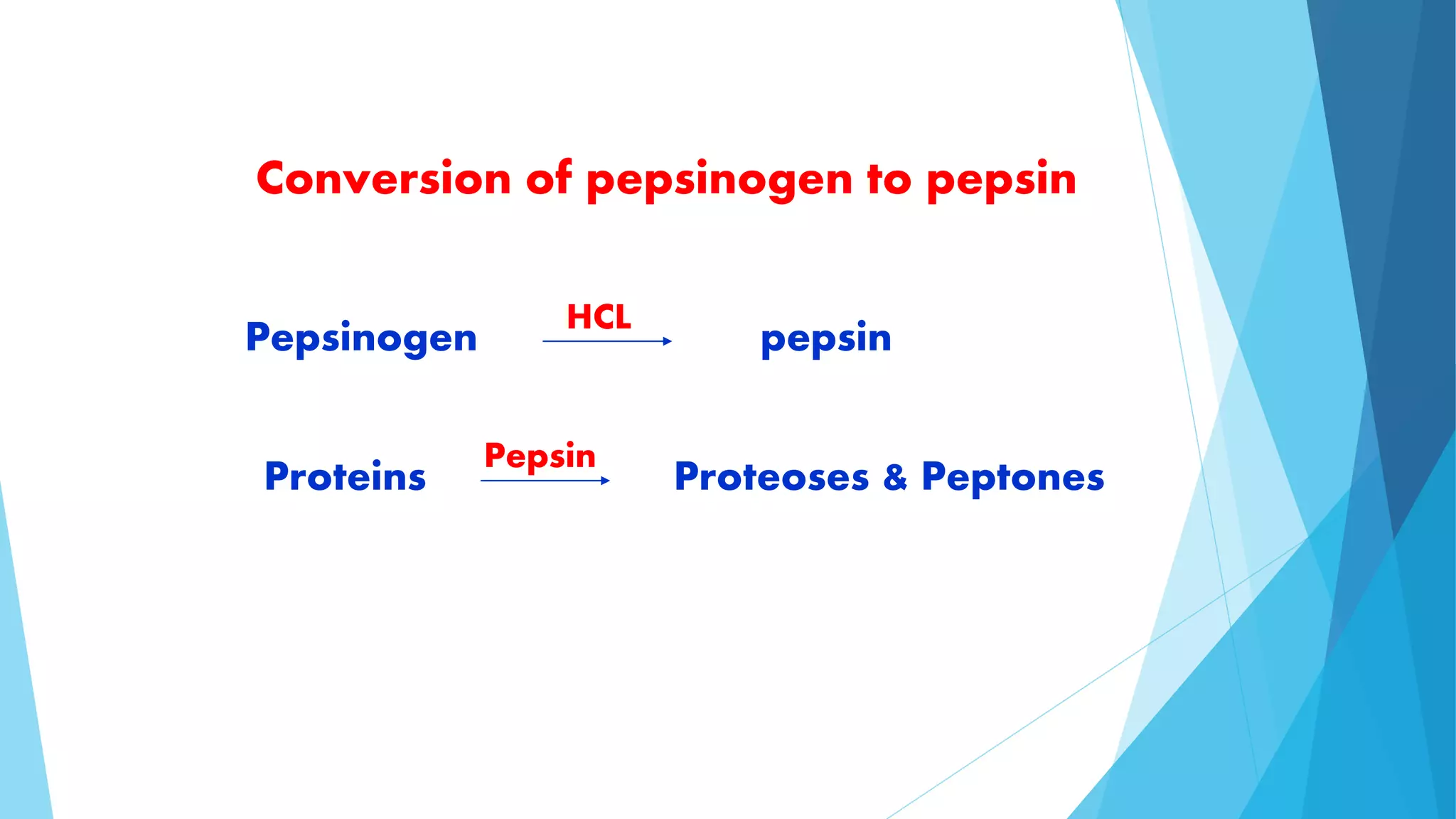
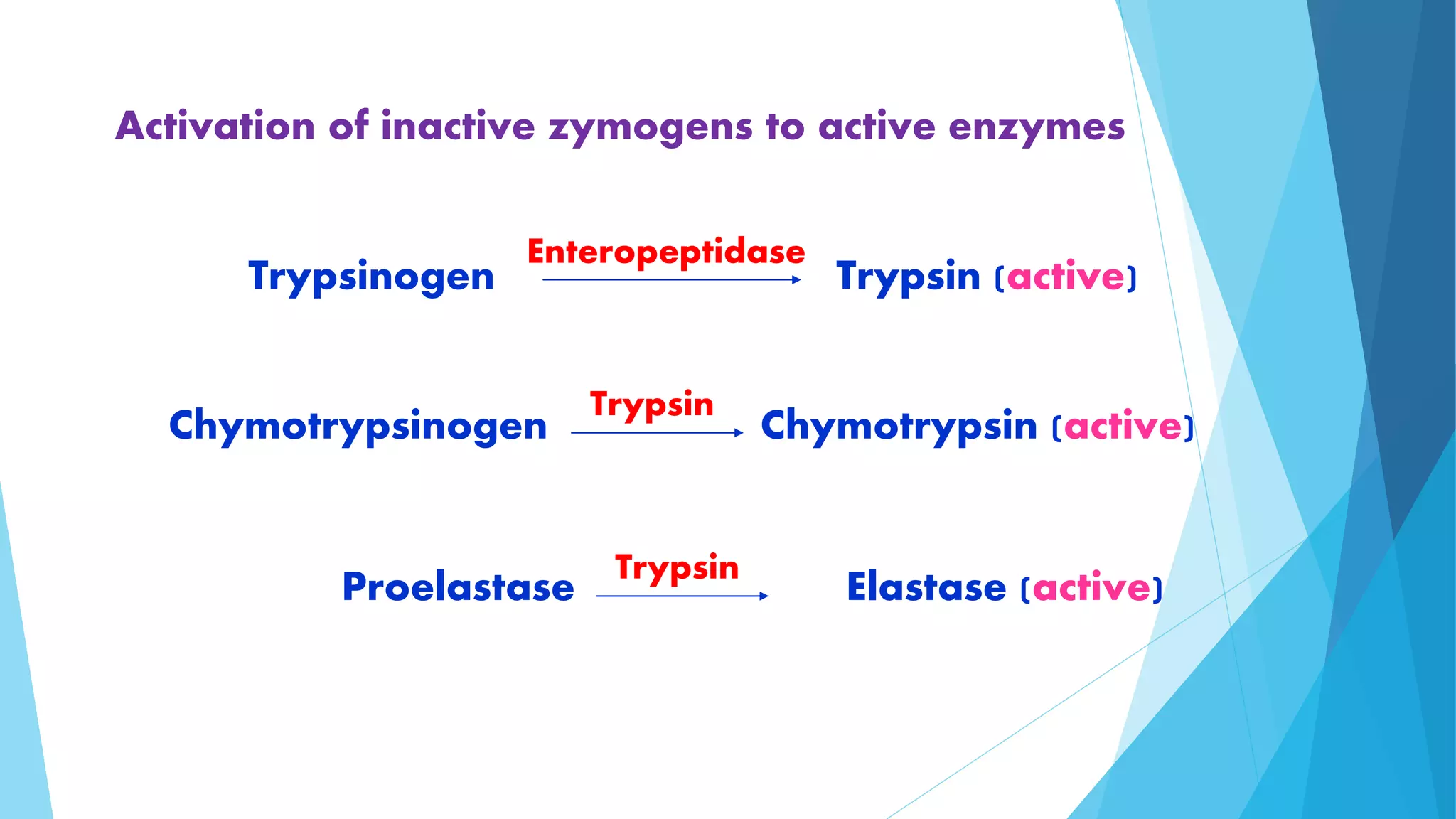
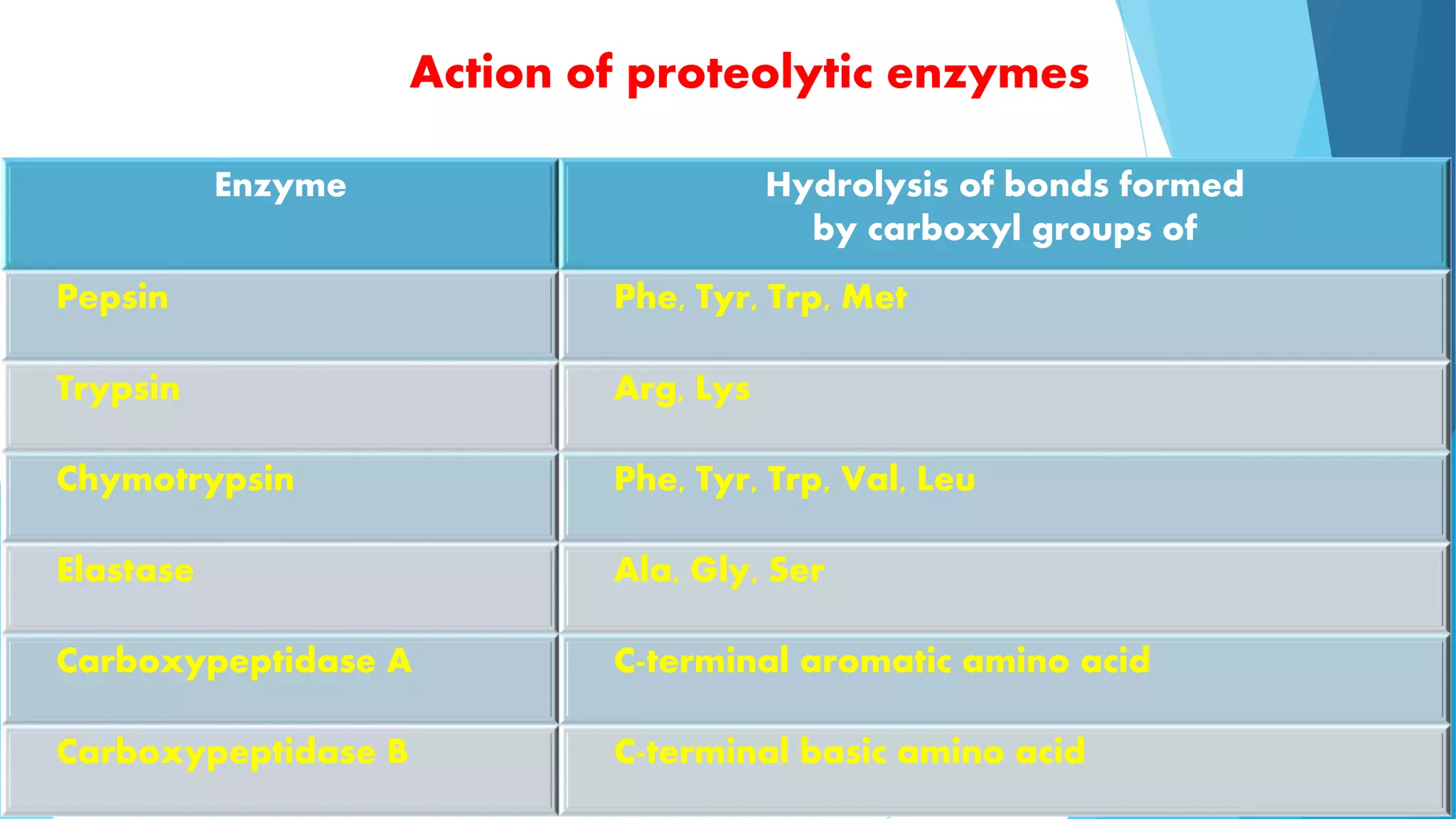
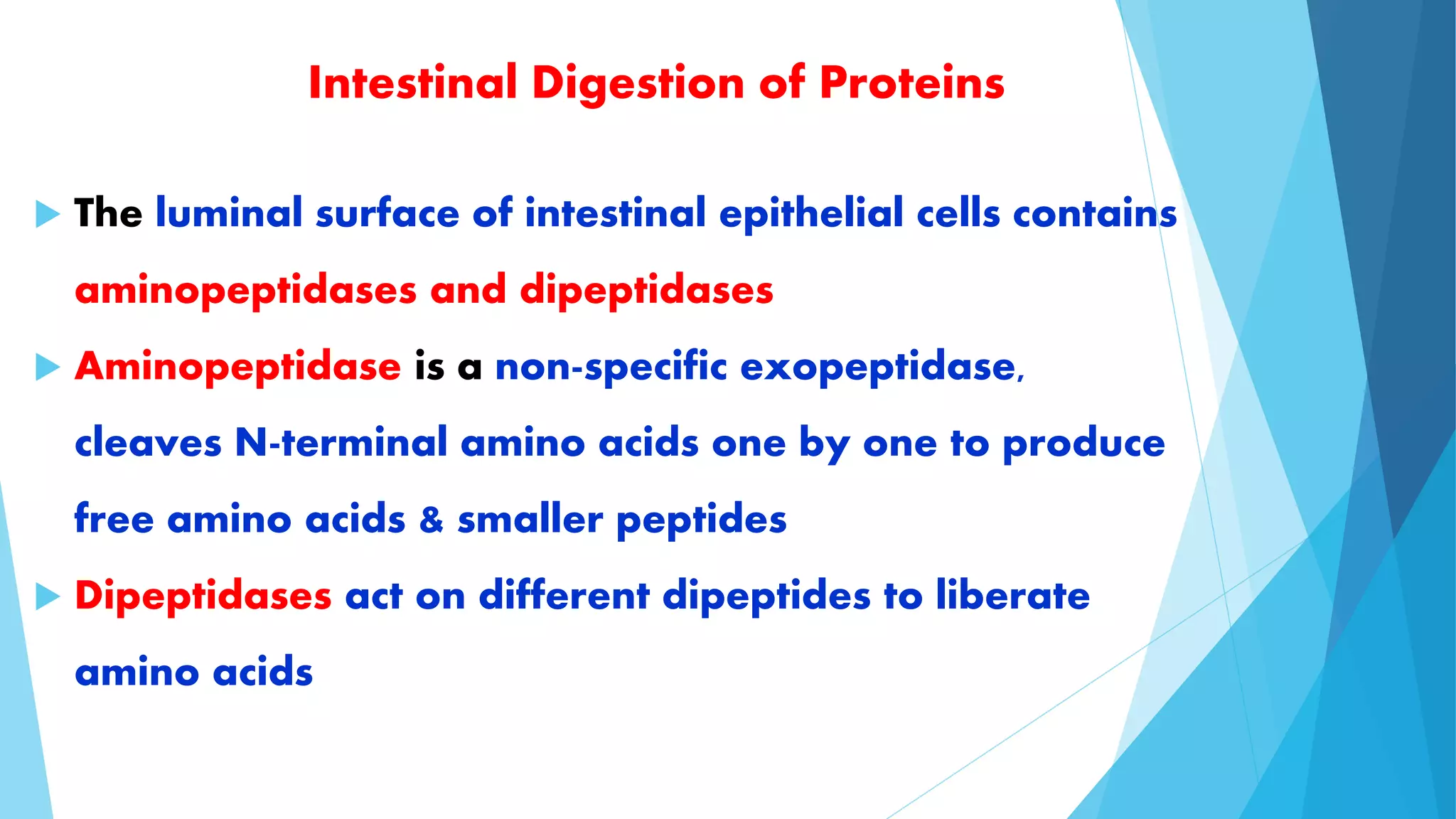
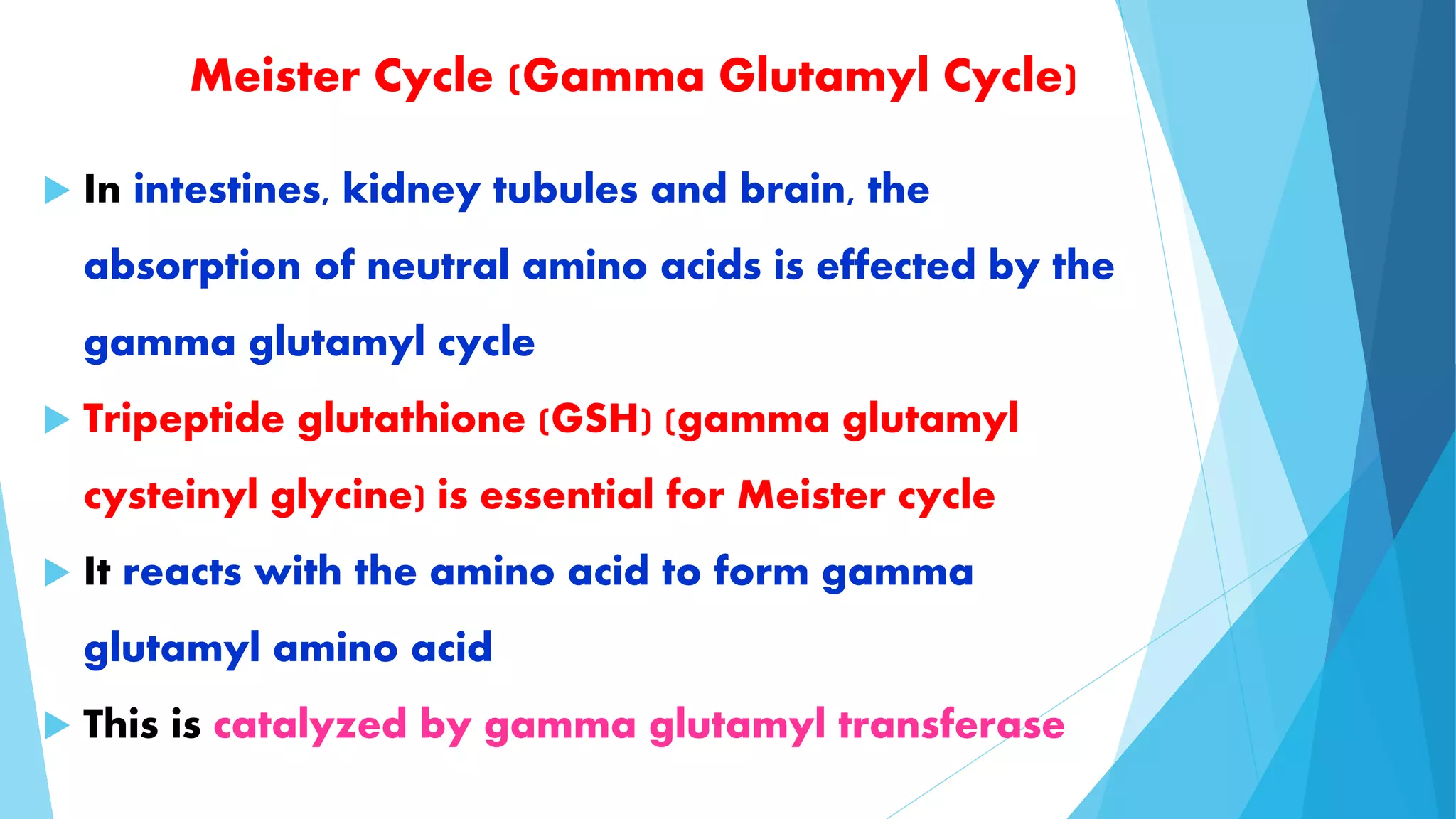
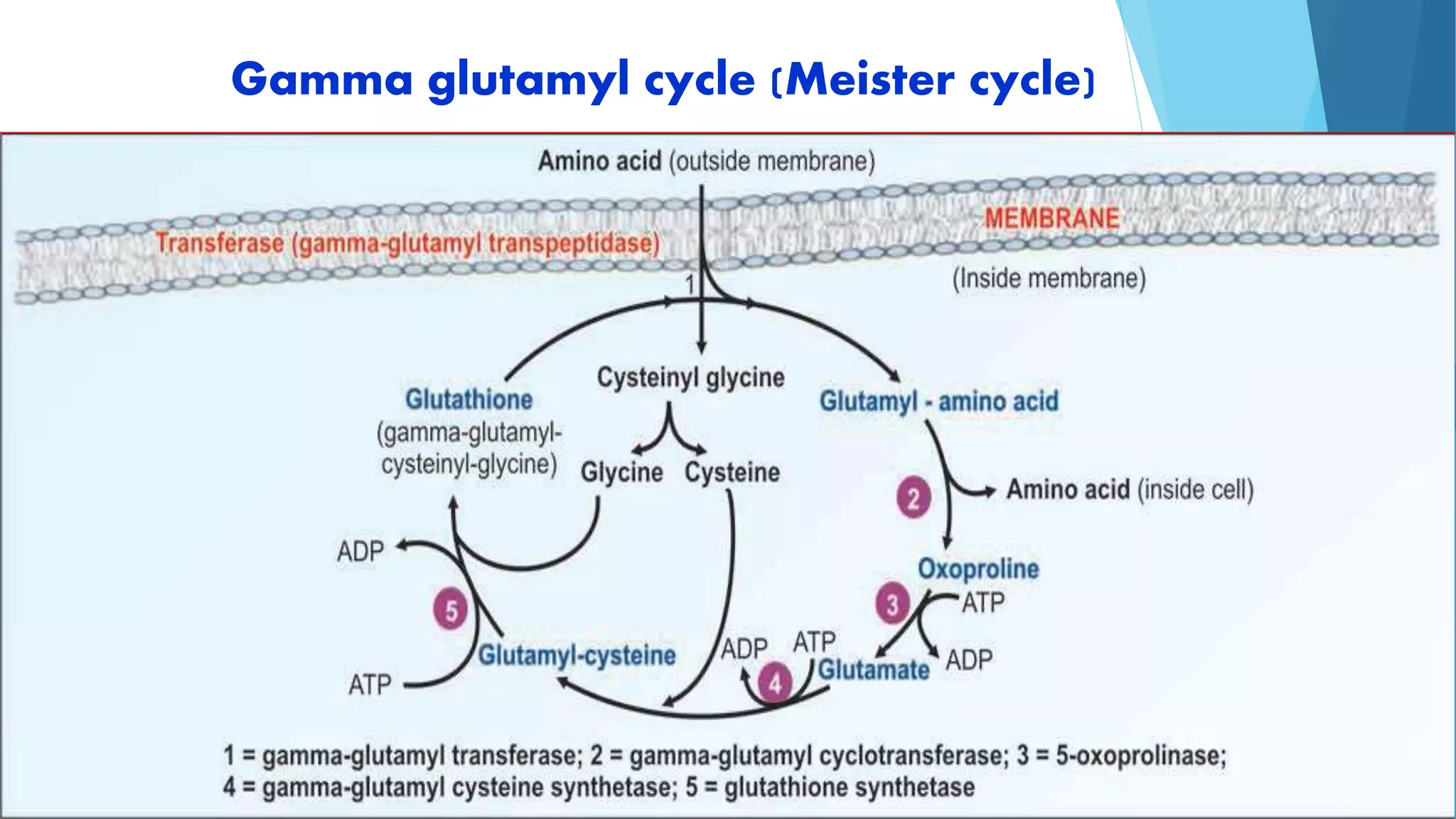
The document summarizes the digestion and absorption of proteins in the human body. Dietary and endogenous proteins are broken down through digestion by enzymes in the stomach, pancreas, and intestines. In the stomach, pepsin digests proteins into proteoses and peptones. The pancreas secretes trypsin, chymotrypsin, and other enzymes as zymogens which are activated and further break down proteins. In the intestines, aminopeptidases and dipeptidases break down peptides into amino acids, which are then absorbed into the bloodstream through active transport mechanisms.