Embed presentation
Download to read offline


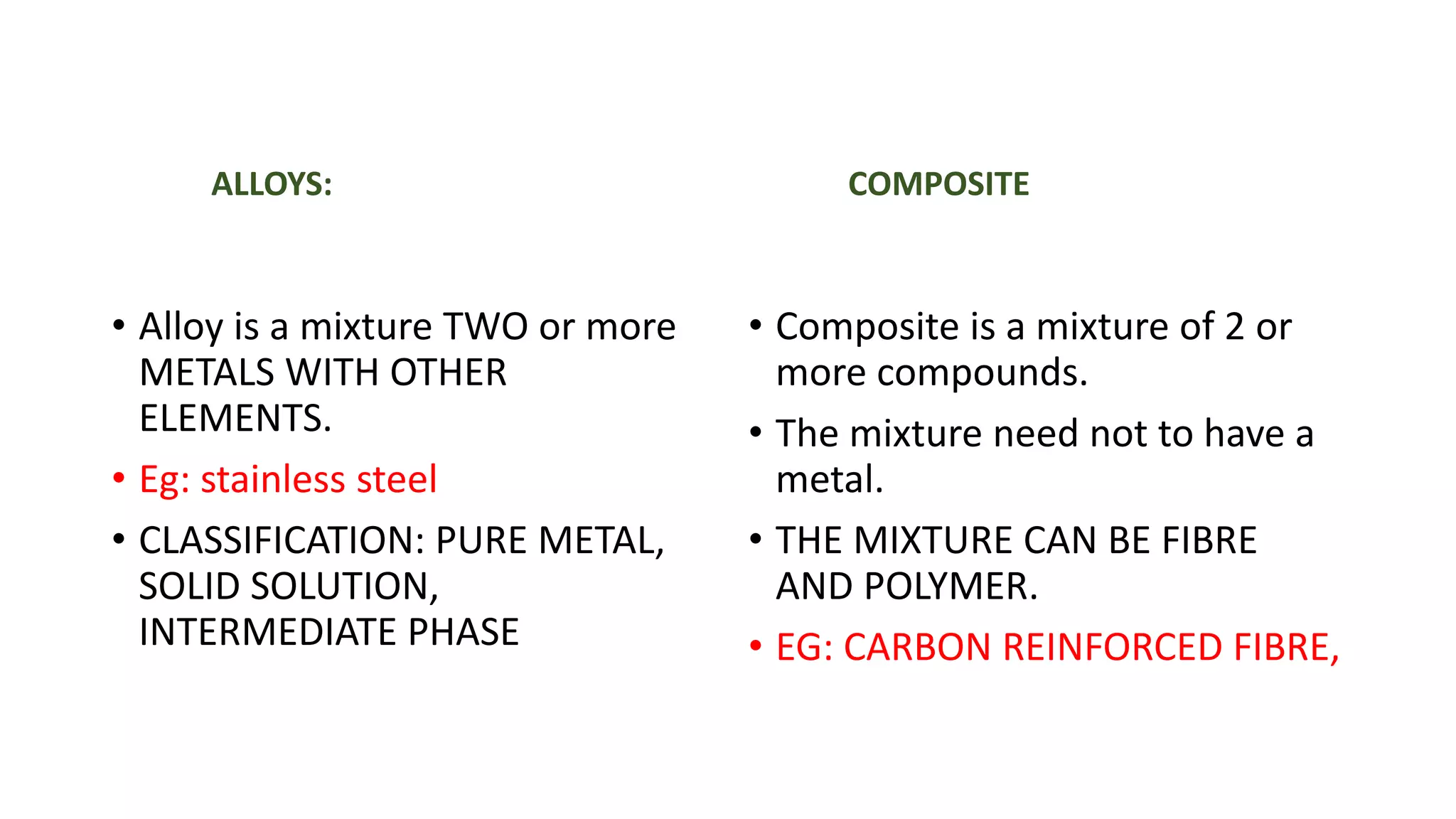

Allotropes refer to different structural forms of the same element, such as diamond and graphite both being made of carbon but having different atomic arrangements. Polymorphism describes how a compound can form different crystal structures, like aragonite and calcite which are both calcium carbonate. Alloys are mixtures of two or more metals combined with other elements, whereas composites combine two or more compounds that may or may not include metals. Solid solutions occur when solid elements dissolve into a single crystal structure completely, such as tin dissolving into copper to form bronze. Phases refer to substances with the same chemical formula but different physical structures, such as water and ice or austenite and ferrite steel phases.



