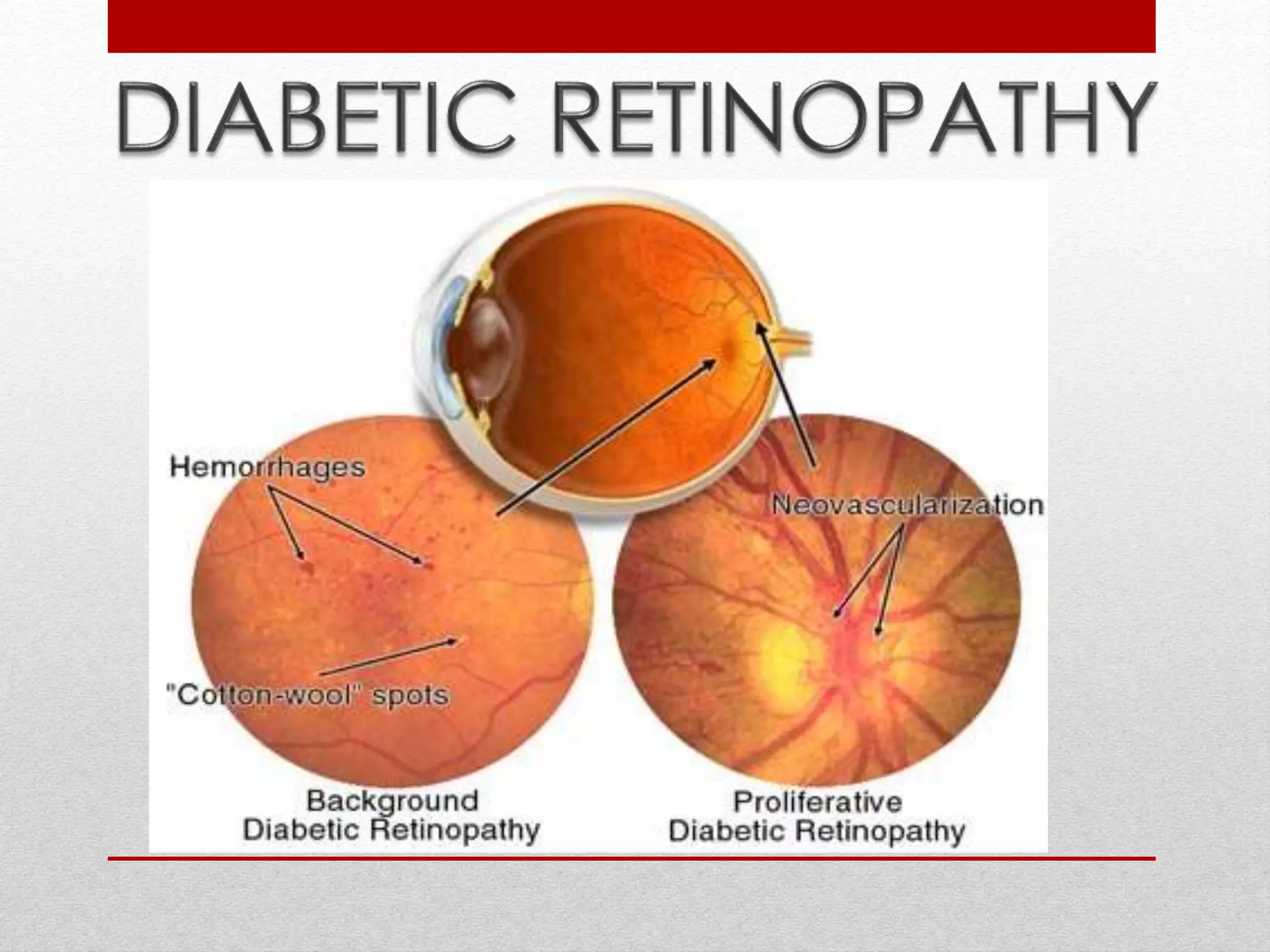
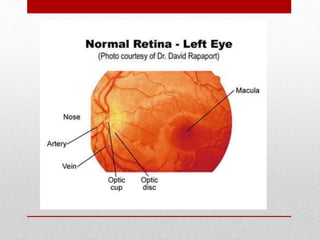
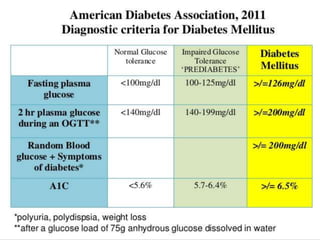
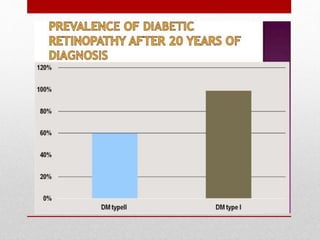
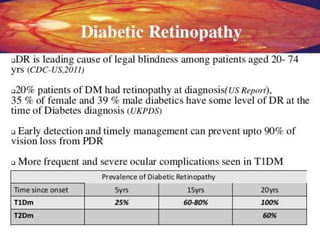
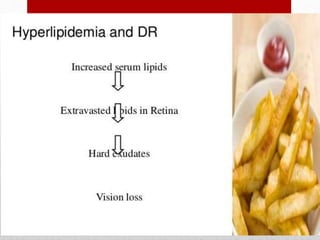
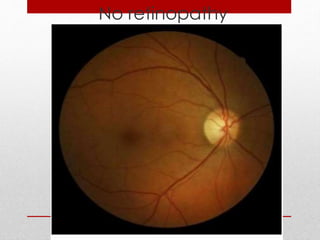
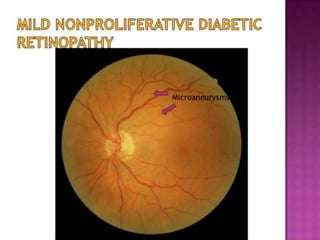
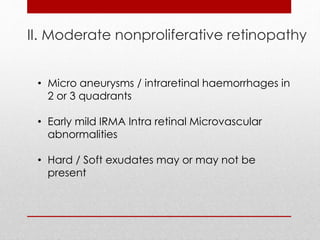
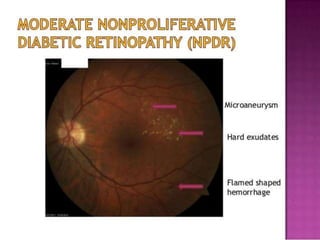
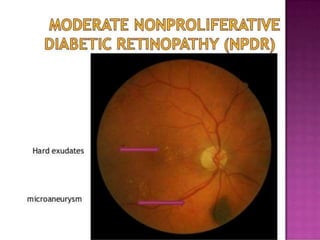
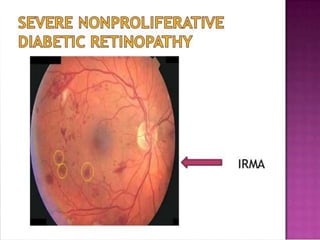
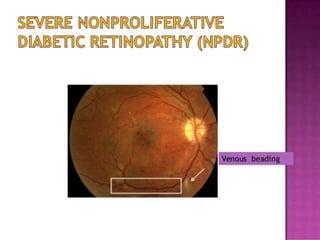
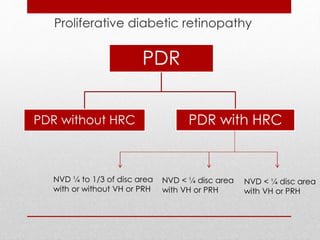
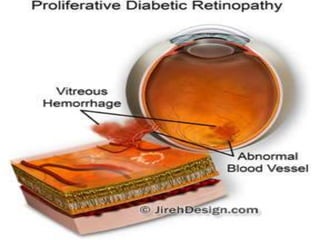
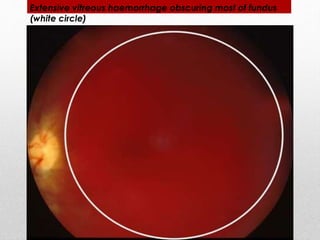
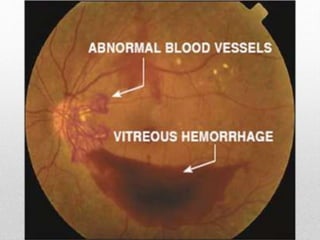
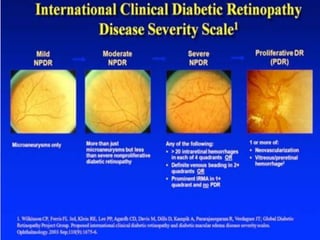
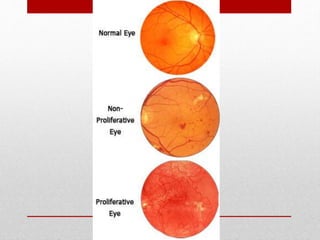
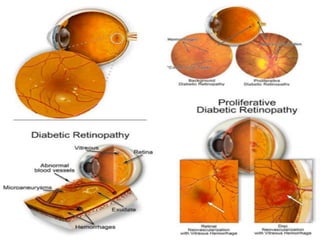
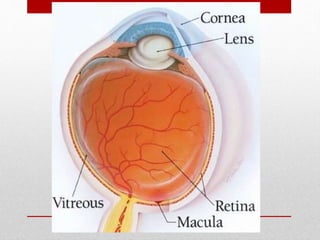
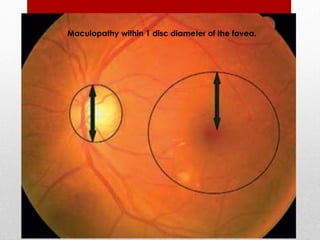
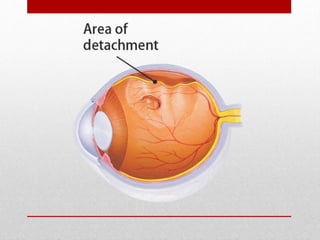
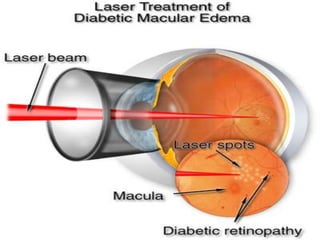
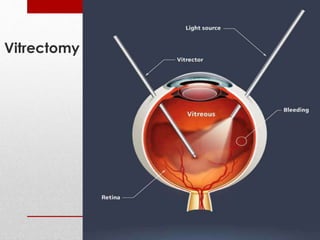
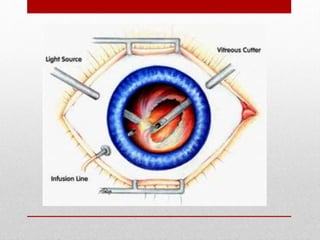
This document discusses diabetic retinopathy, its causes, stages, treatments, and prevention. It is progressive retinal vessel dysfunction caused by long-term hyperglycemia. Key factors that contribute to its development include hypertension, hyperlipidemia, female sex, pregnancy, smoking, obesity, and poor metabolic control. Stages include non-proliferative and proliferative retinopathy. Treatments include anti-VEGF drugs, laser photocoagulation, vitrectomy, and strict control of blood sugar and blood pressure to prevent its progression.