Embed presentation
Downloaded 35 times




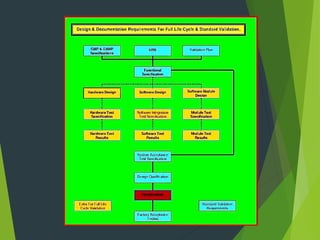






This document discusses design qualification (DQ), which involves planning, decision making, and risk analysis for new equipment before construction. DQ ensures the proposed design of facilities, systems, and equipment is suitable for the intended purpose. It involves teams from engineering, production, quality management, and project personnel. The DQ documentation covers project description, regulatory requirements, facility and environmental requirements, utilities, technical specifications, materials, cleaning procedures, performance data, controls, calibration, and maintenance.









