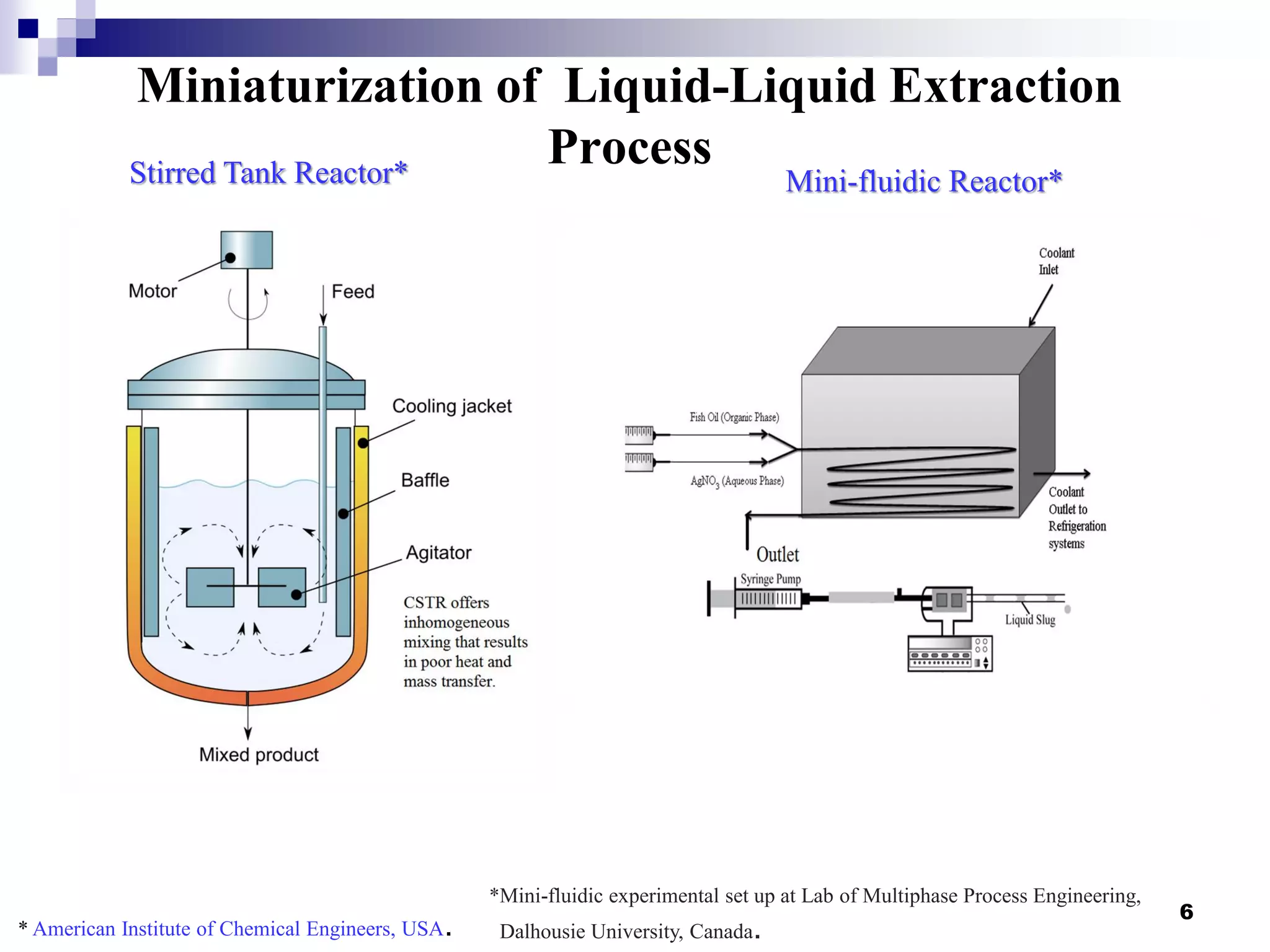
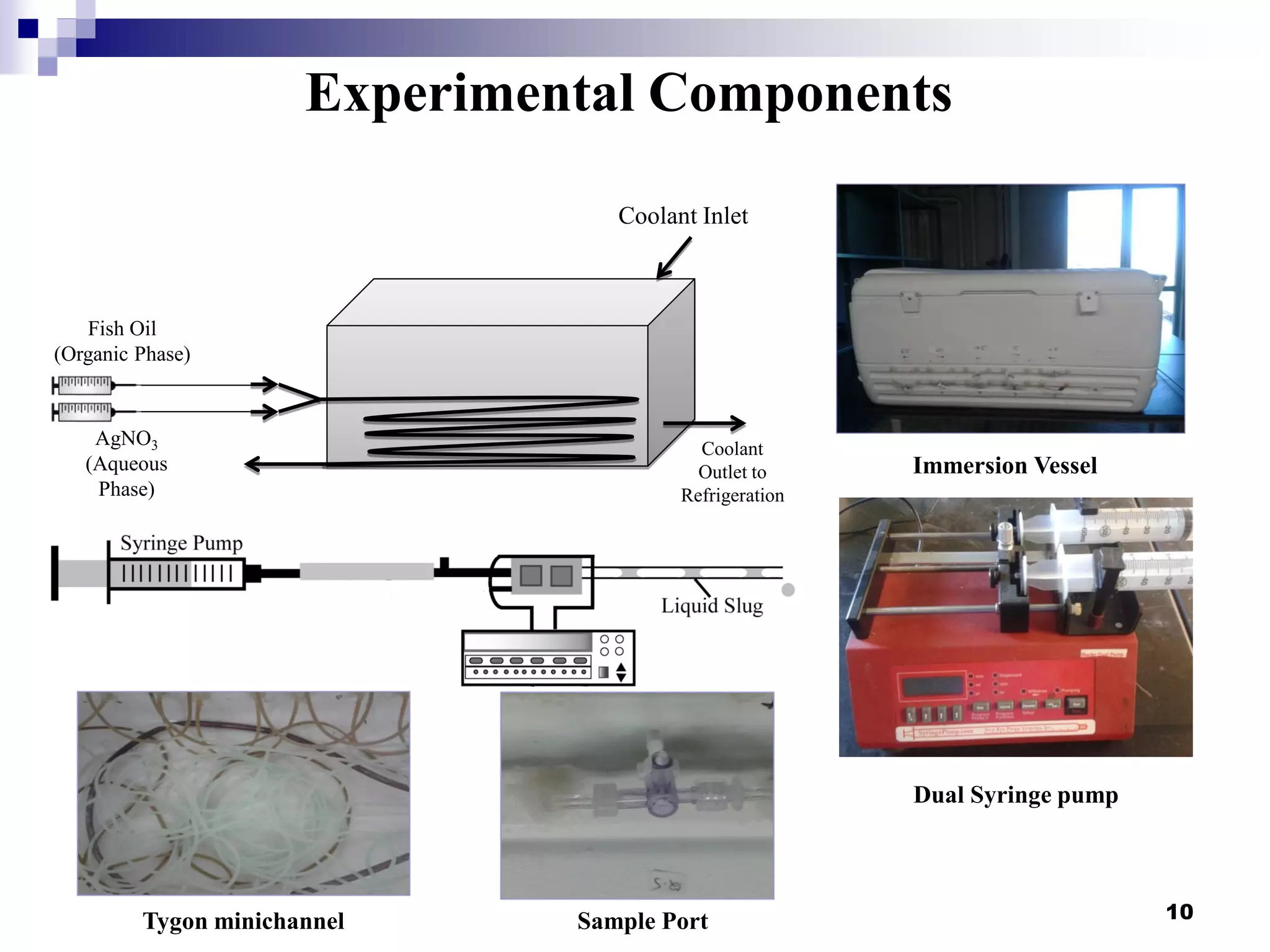
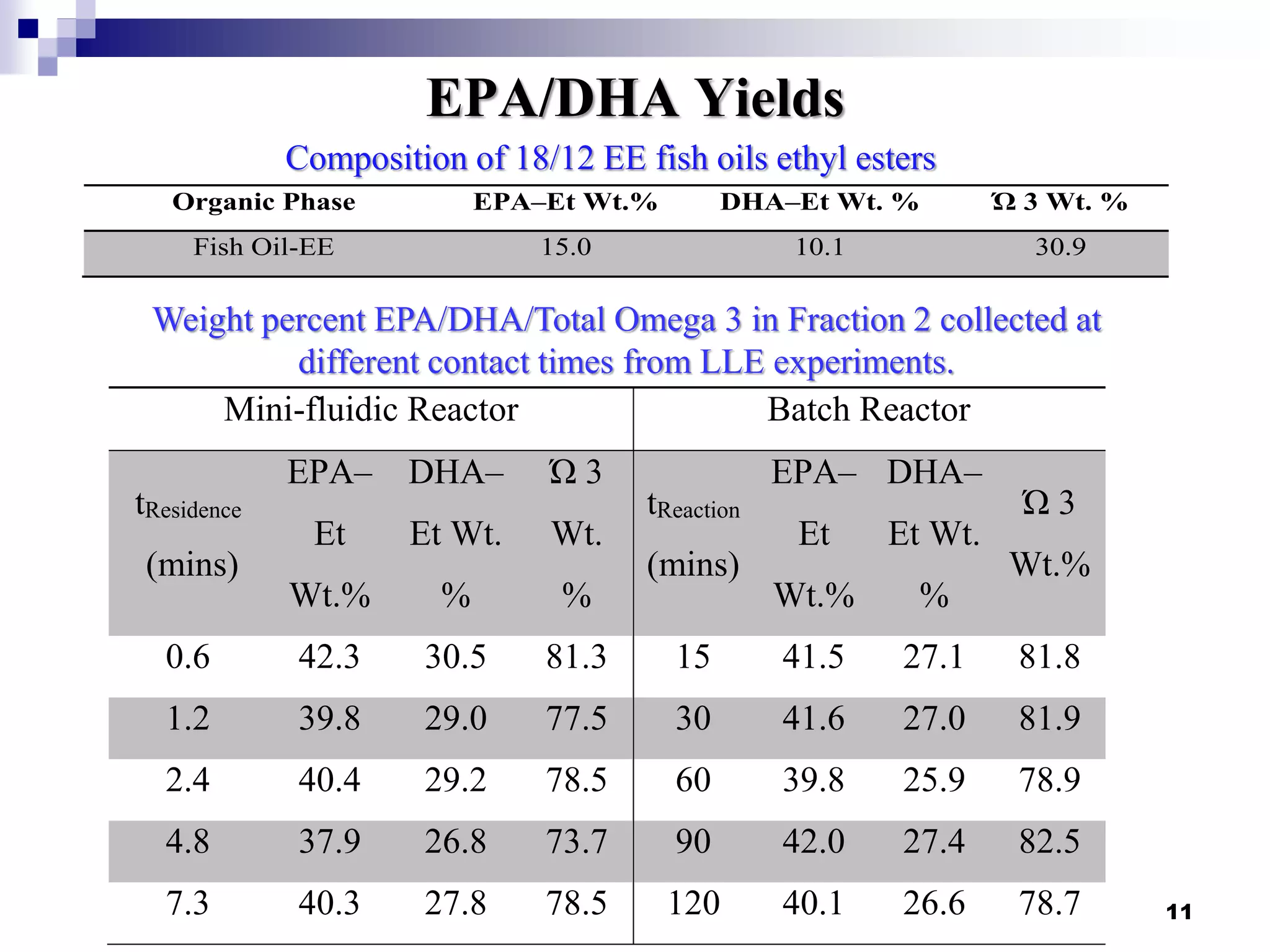
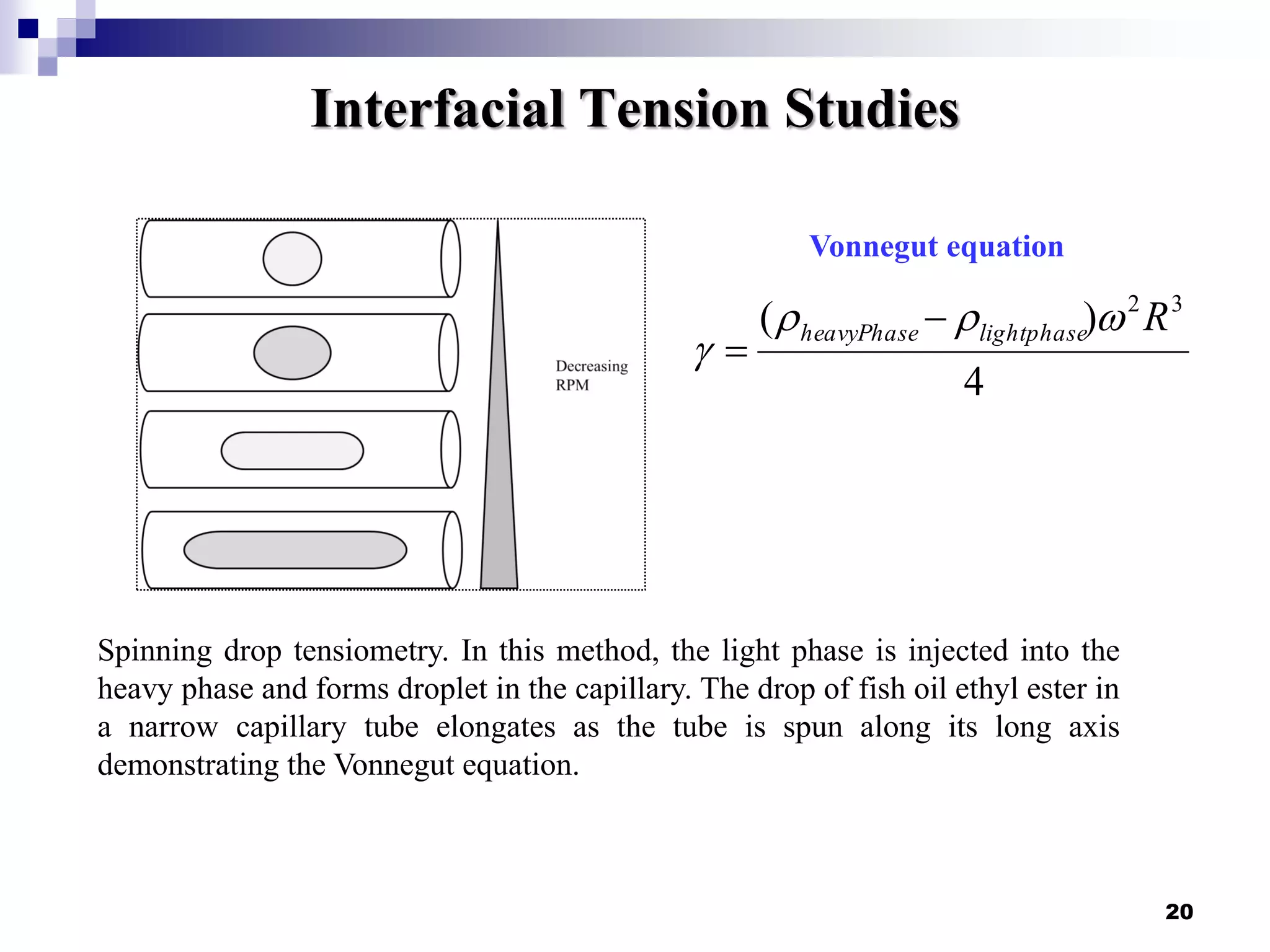
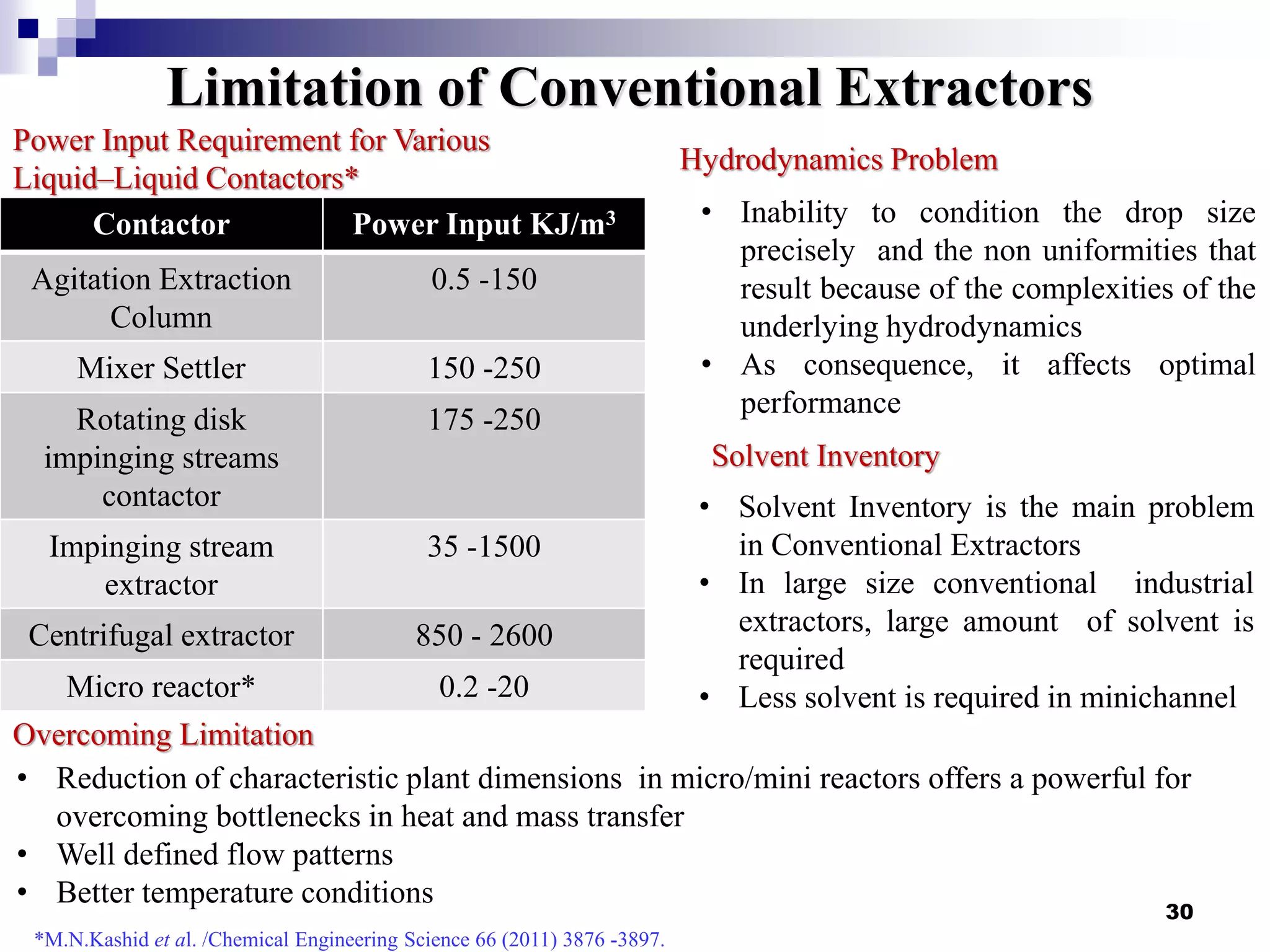
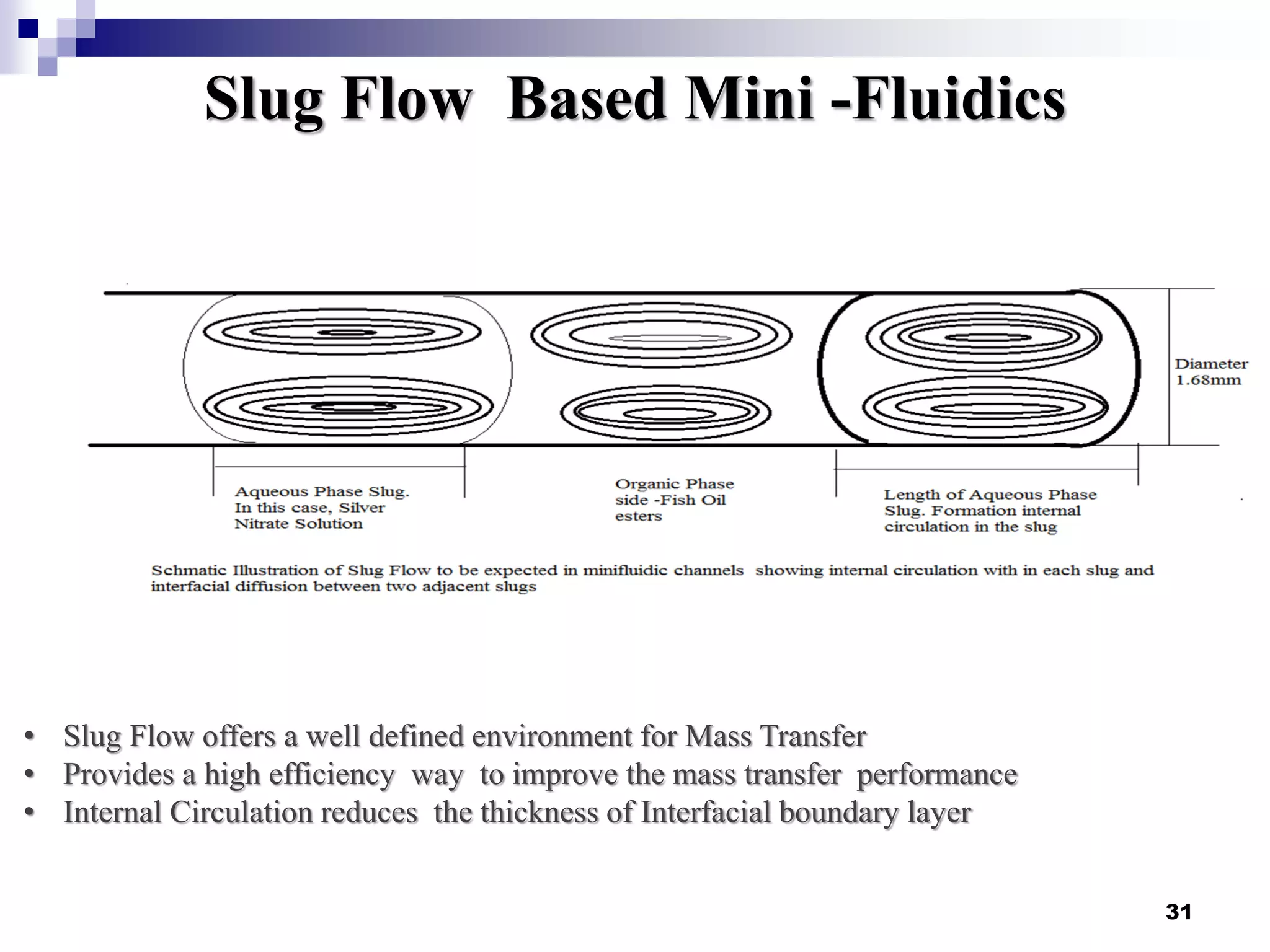
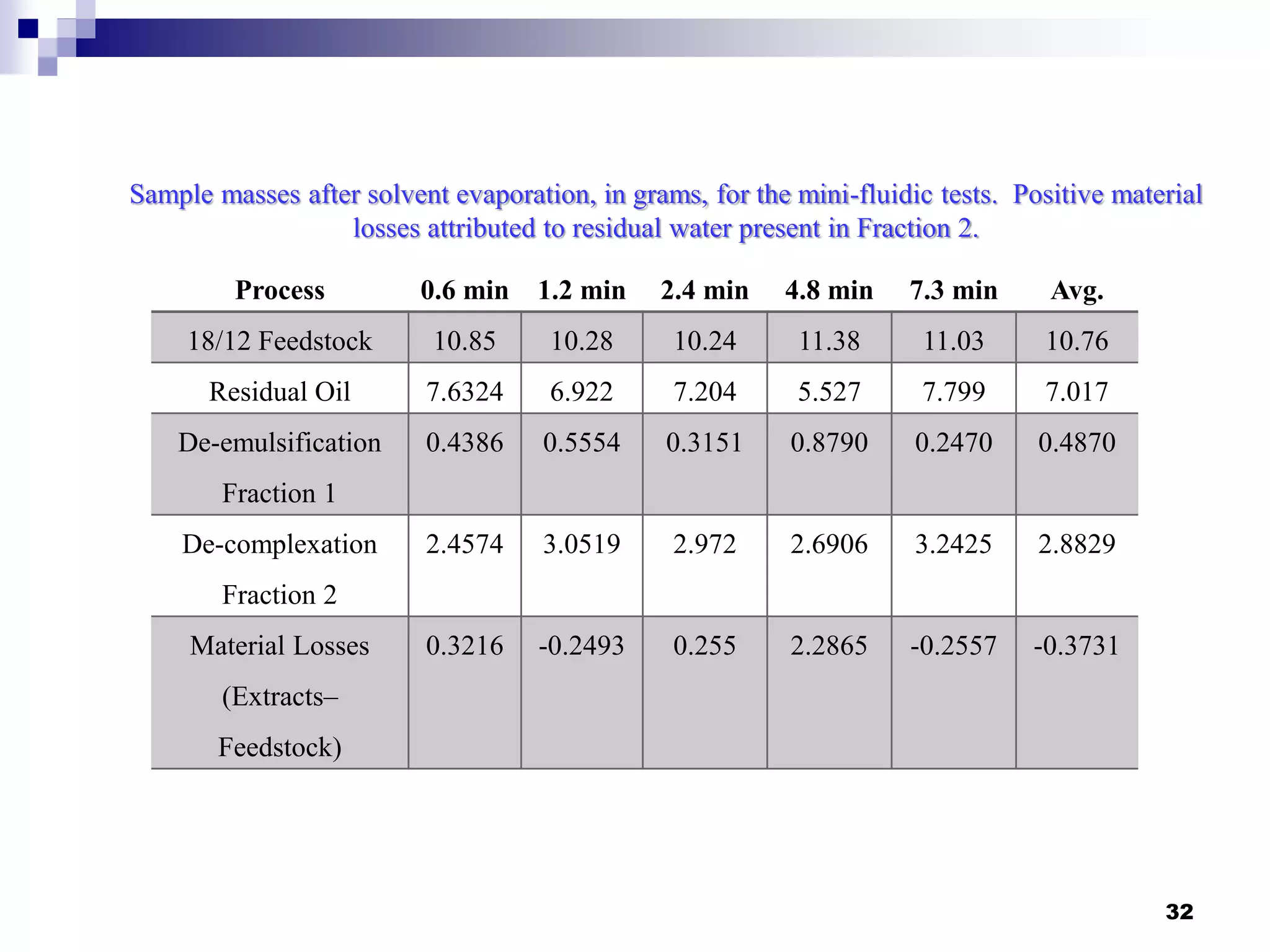
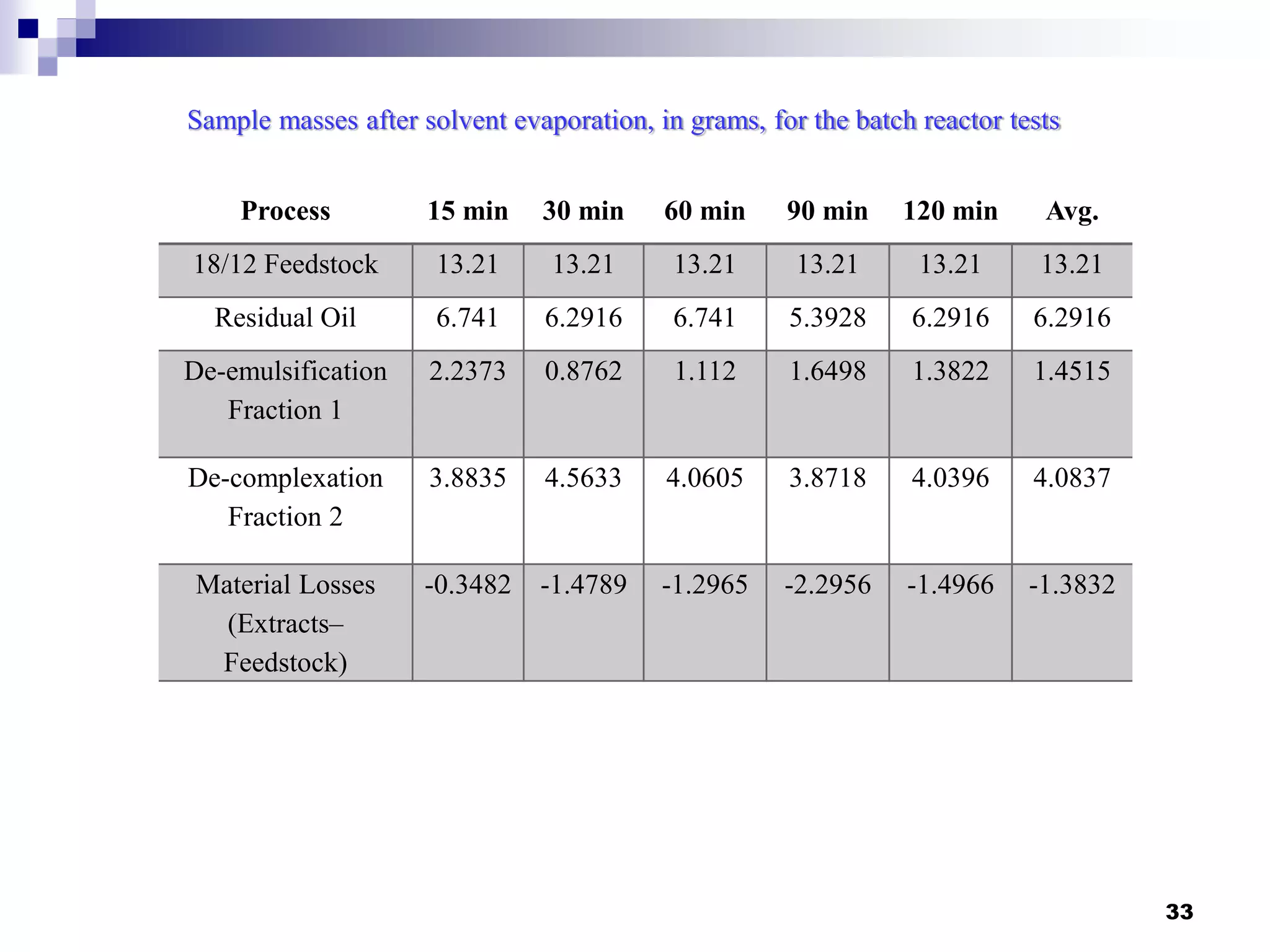
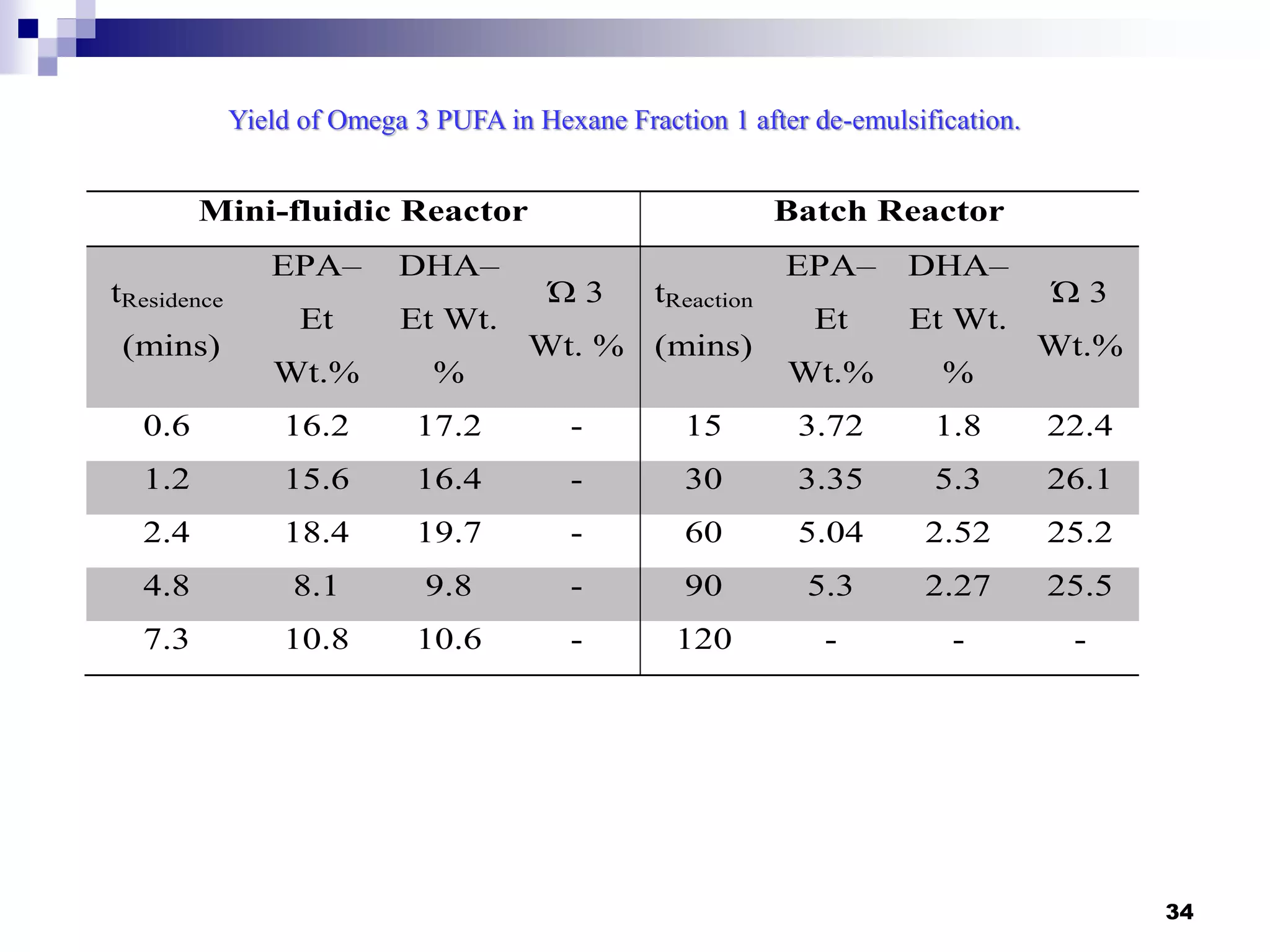
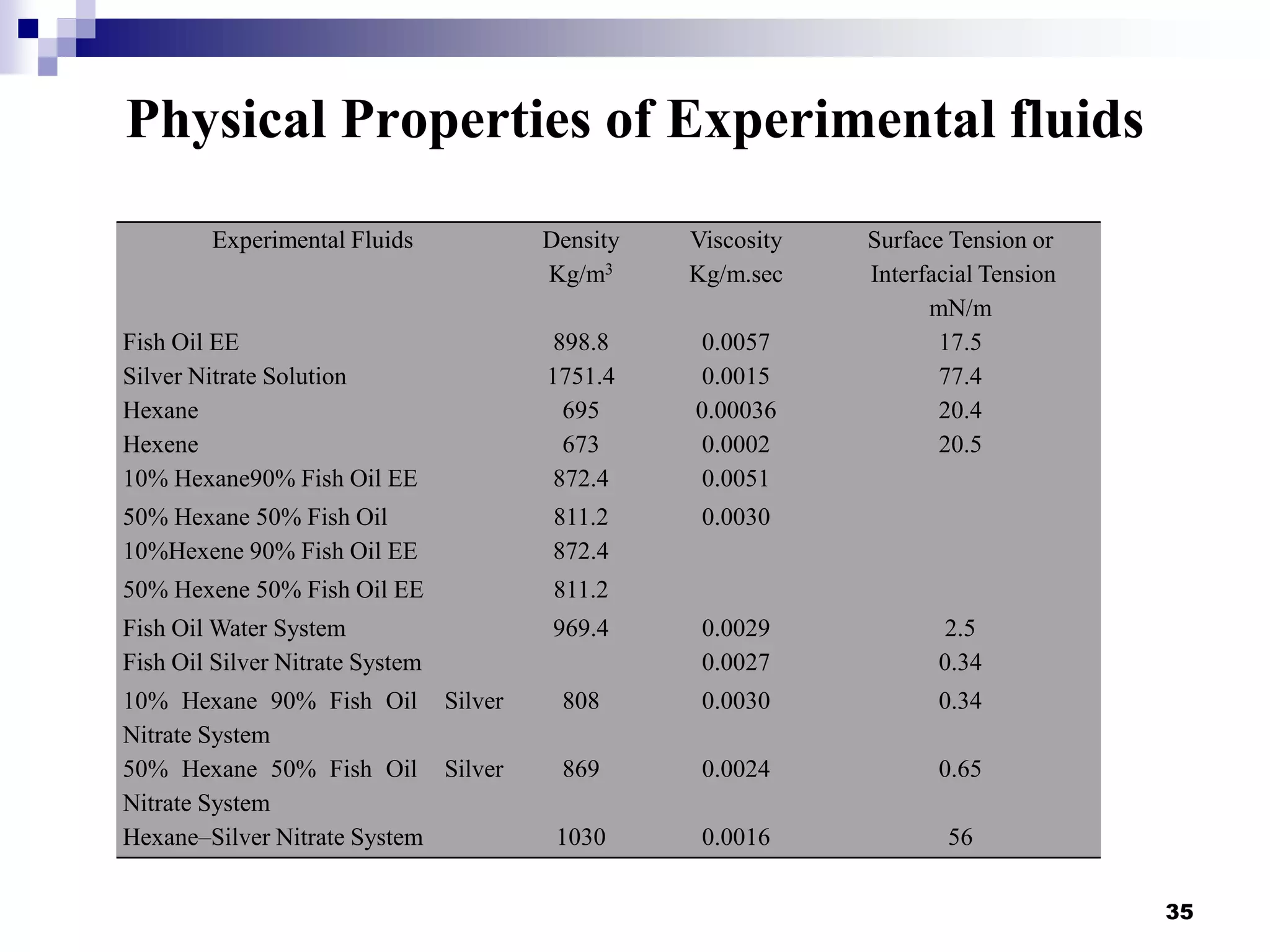
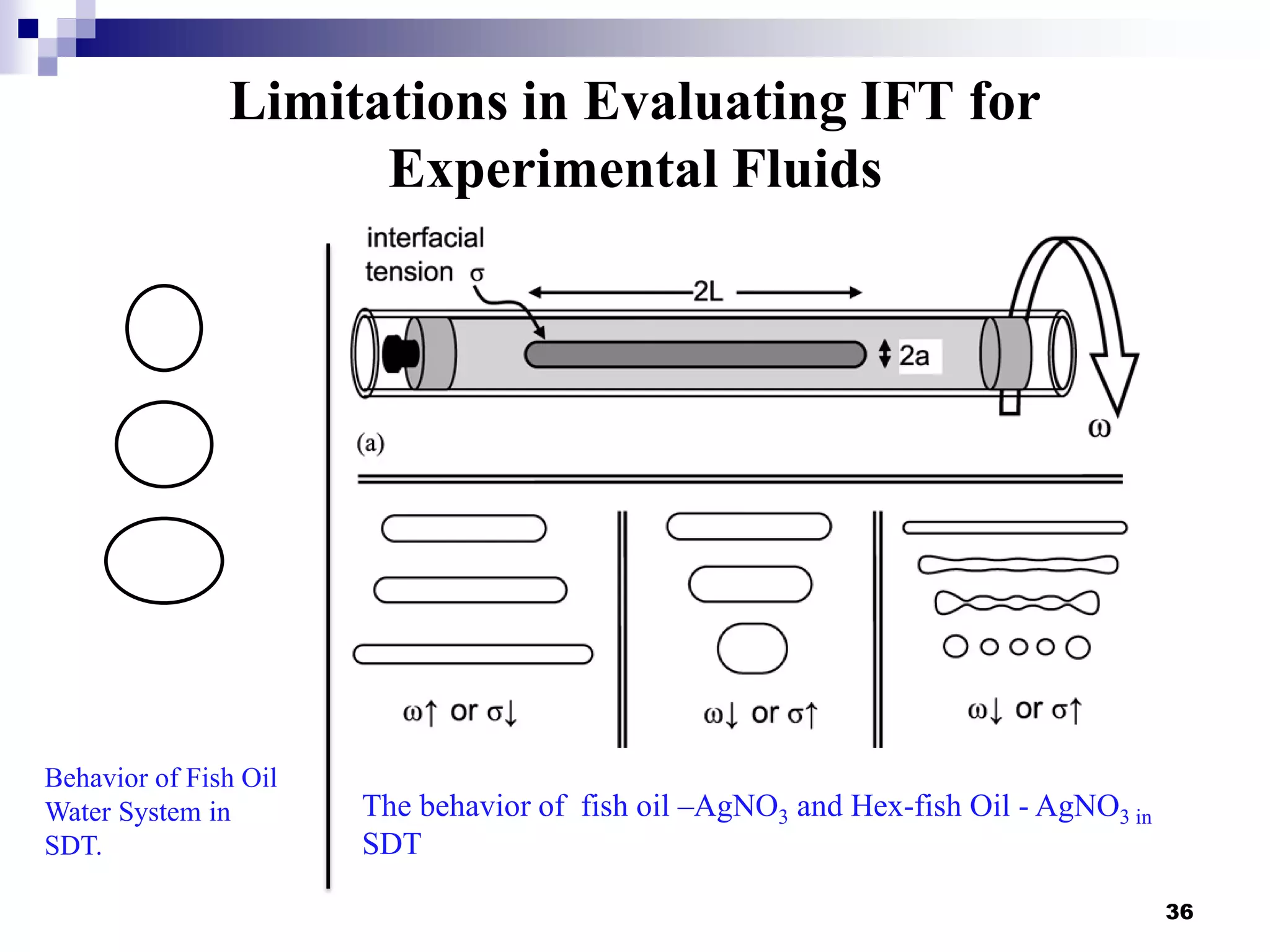
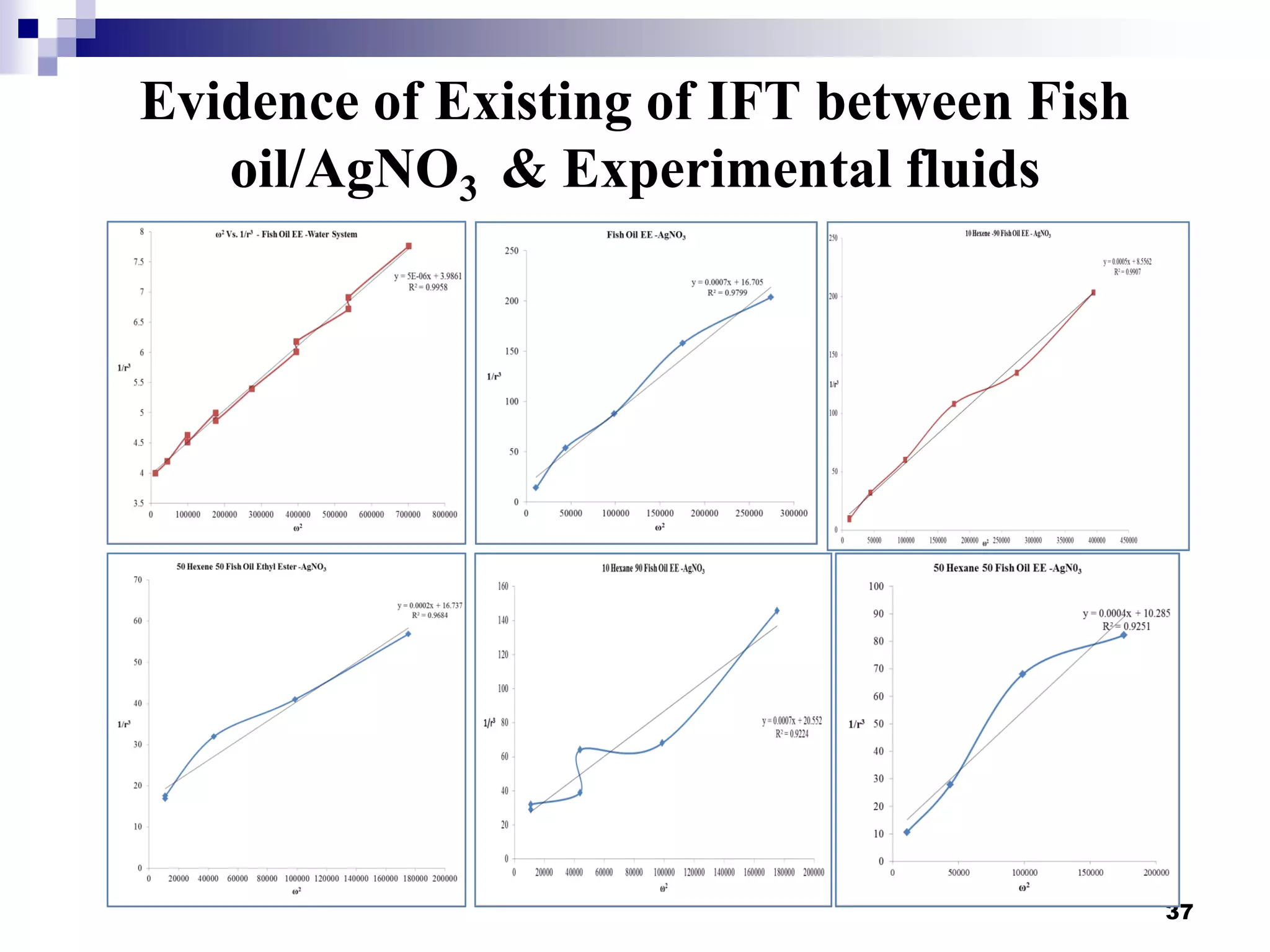
The document describes research on extracting EPA/DHA from fish oil using a mini-fluidic reactor and comparing it to a batch reactor. Key findings include:
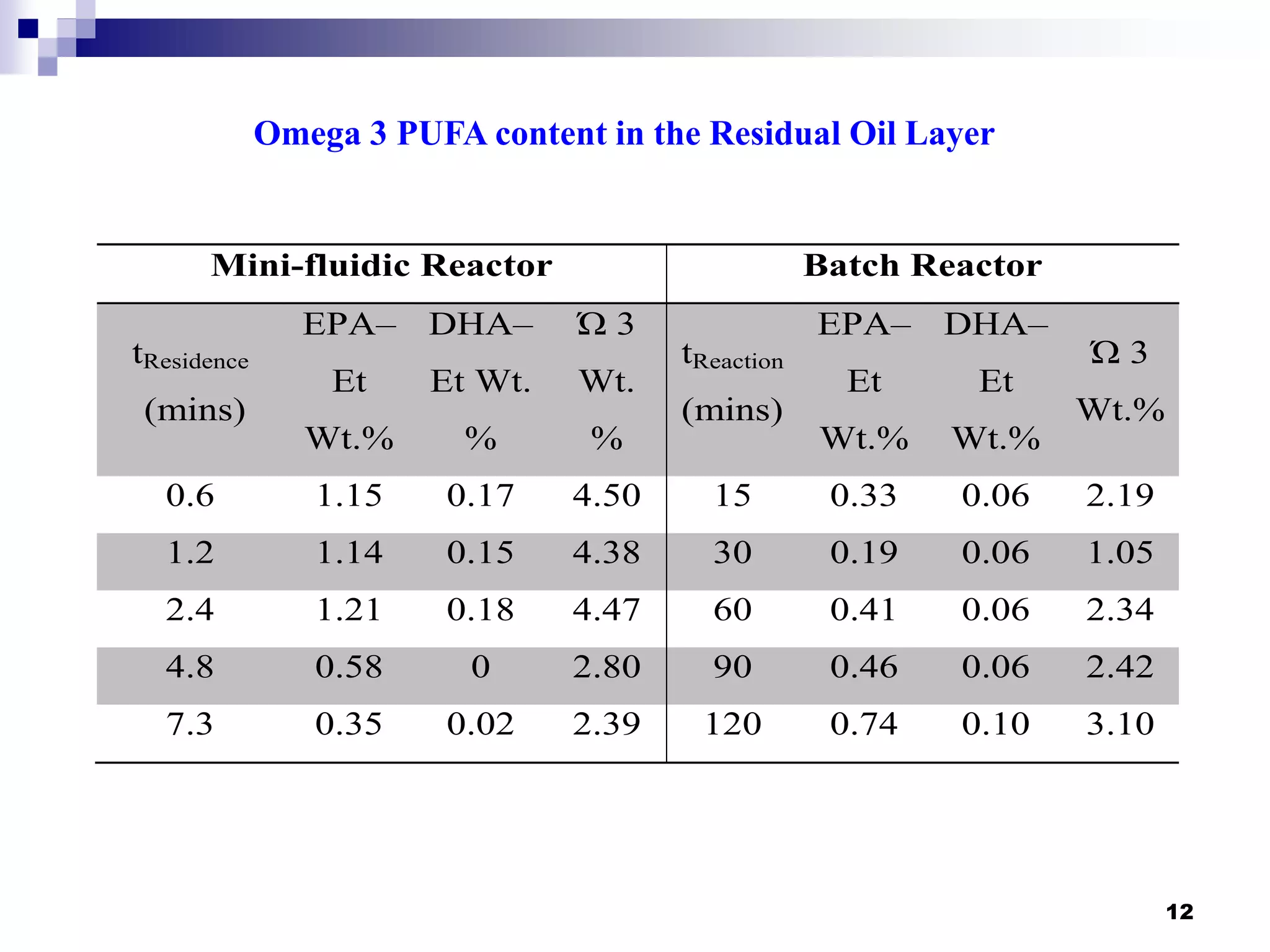
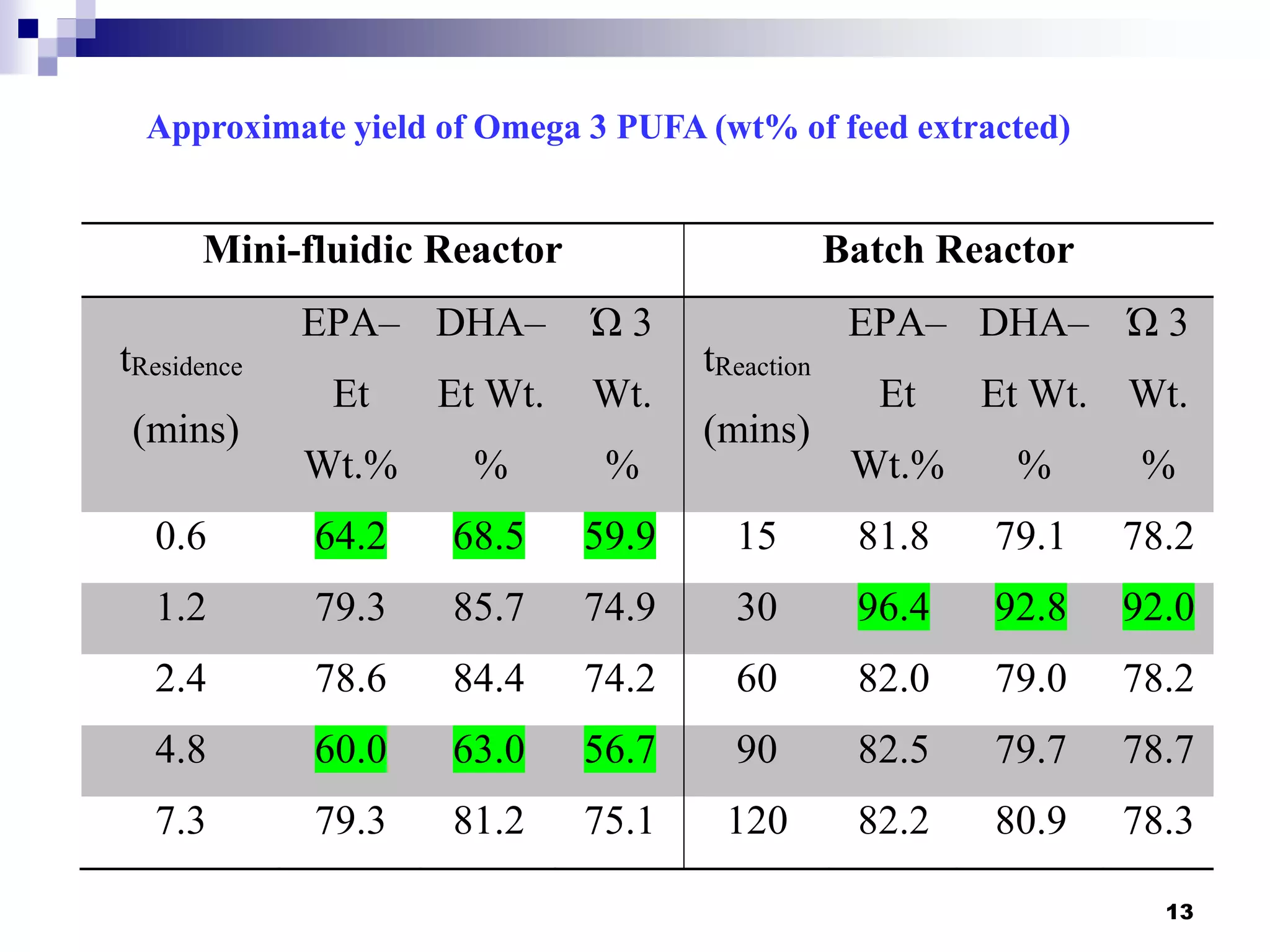
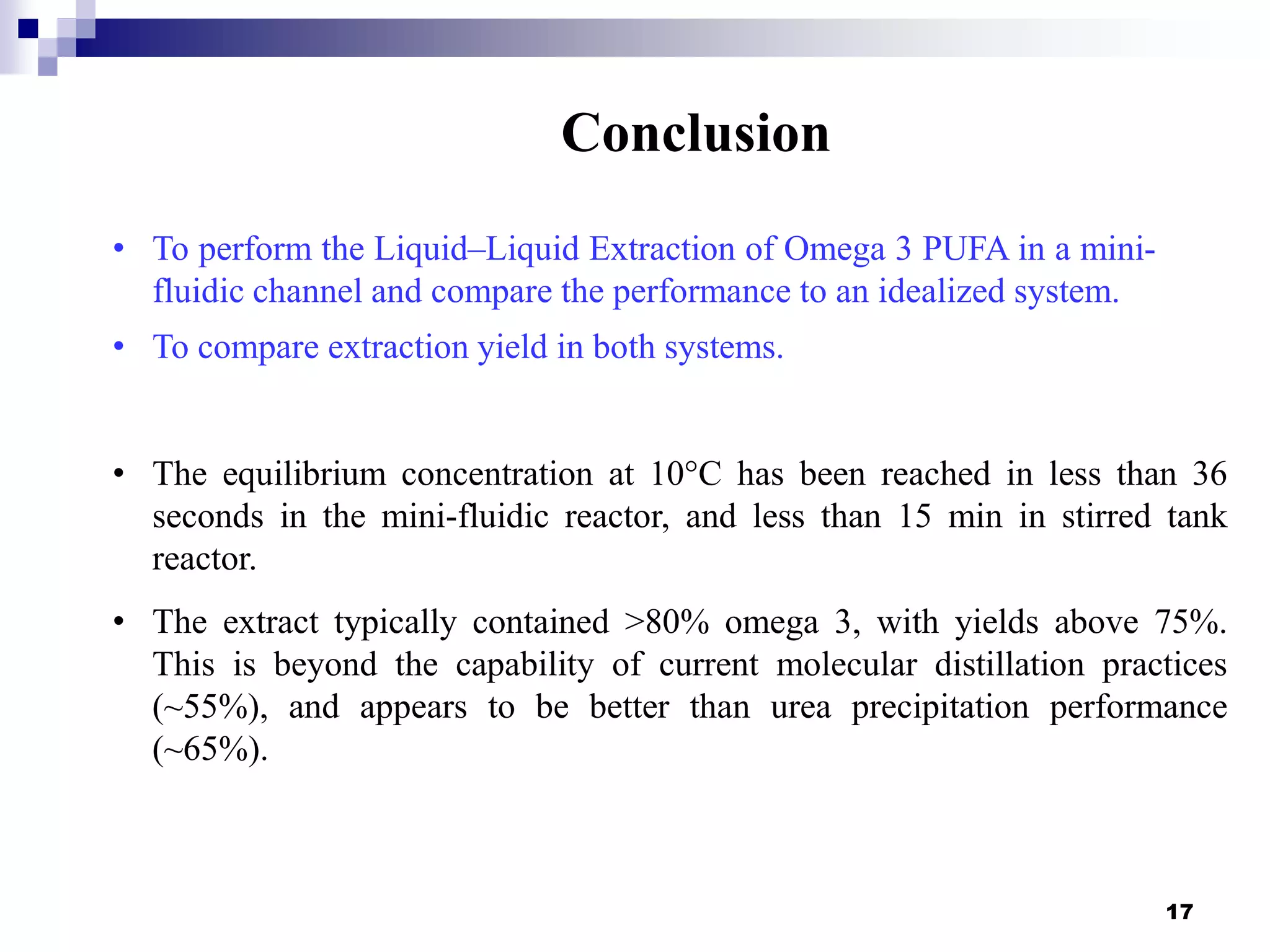
- The mini-fluidic reactor reached equilibrium concentration at 10°C in less than 36 seconds, while the batch reactor took over 15 minutes. Both systems extracted over 75% of omega-3 fatty acids from the fish oil feedstock.
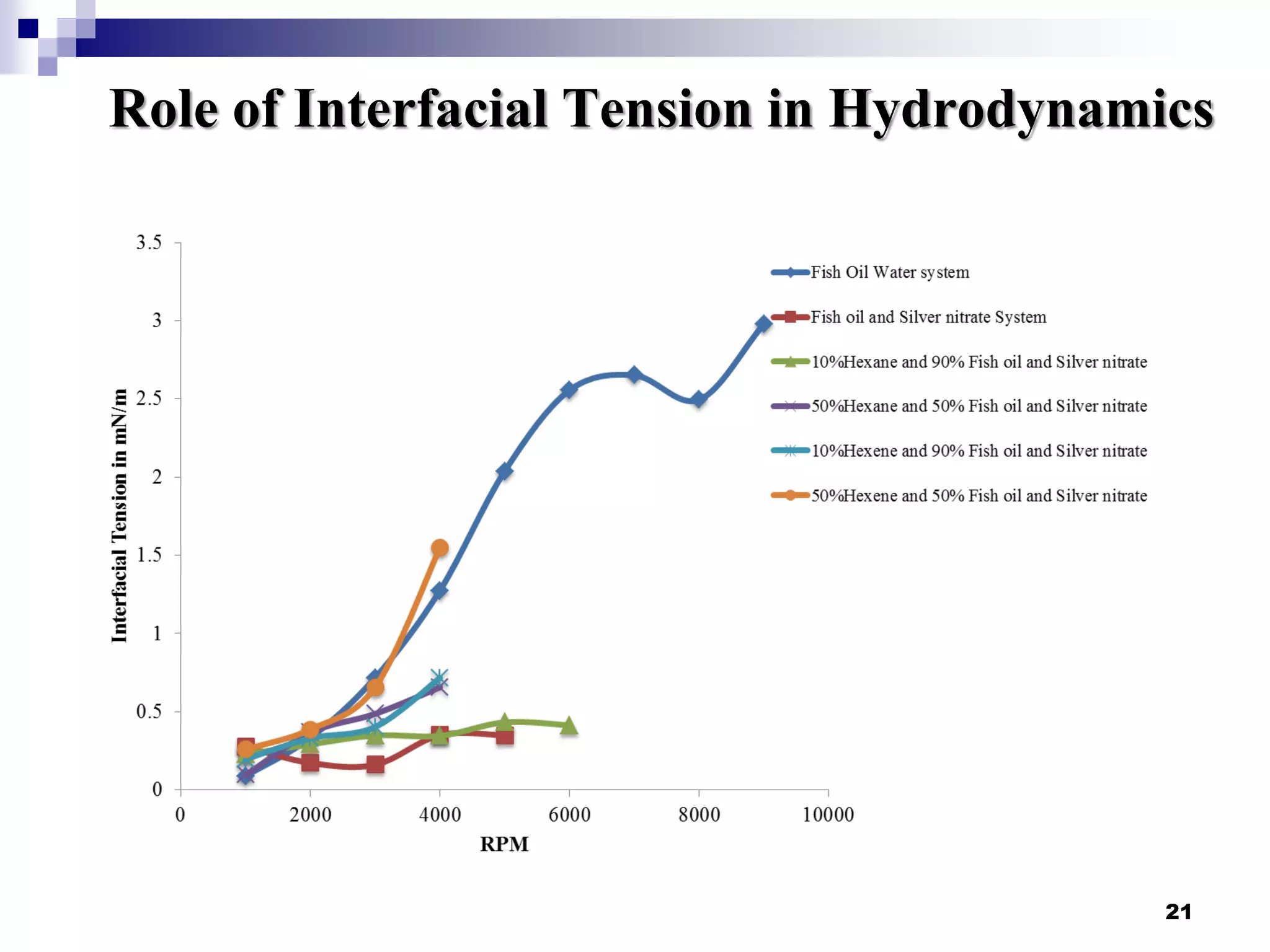
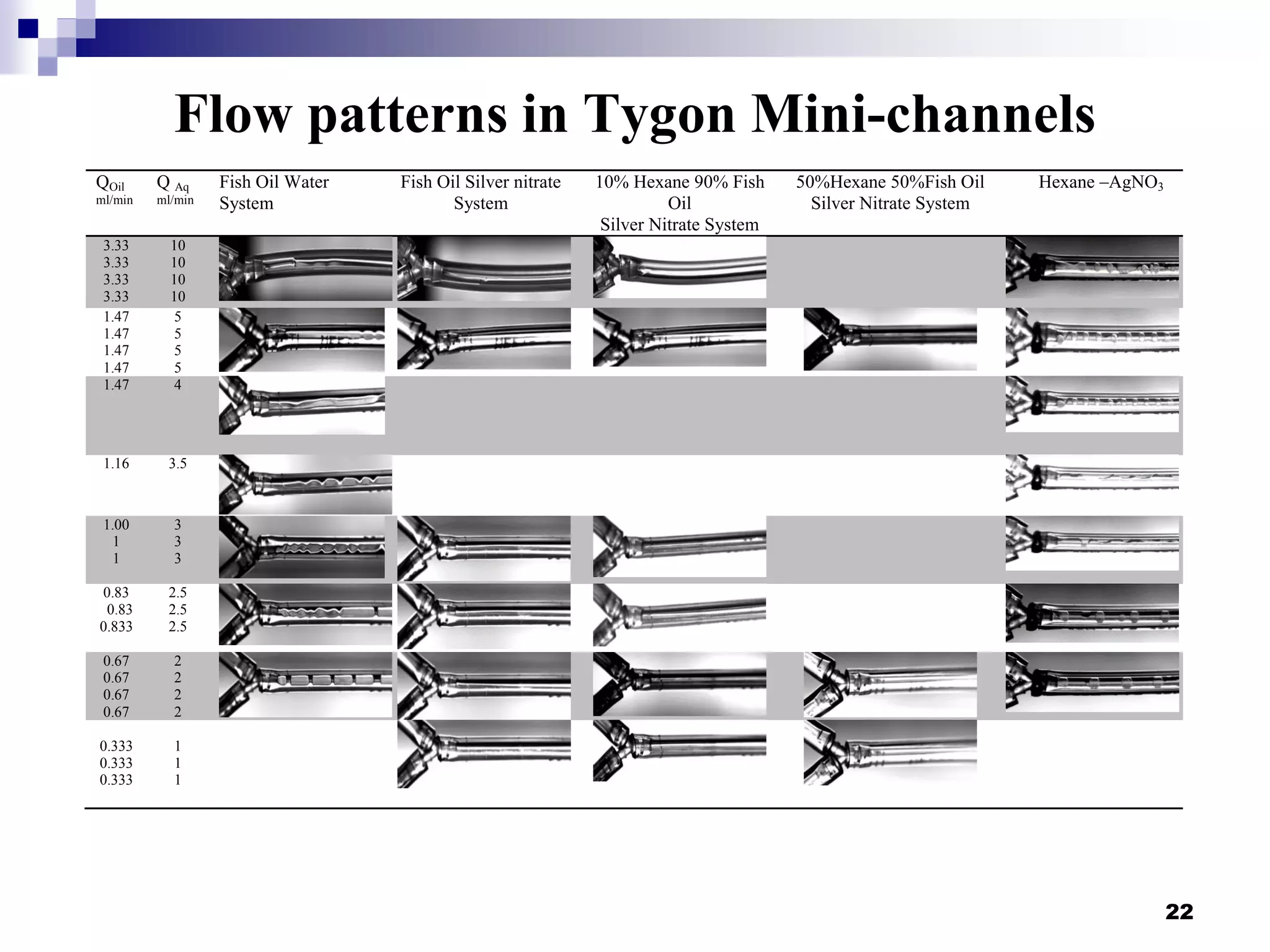
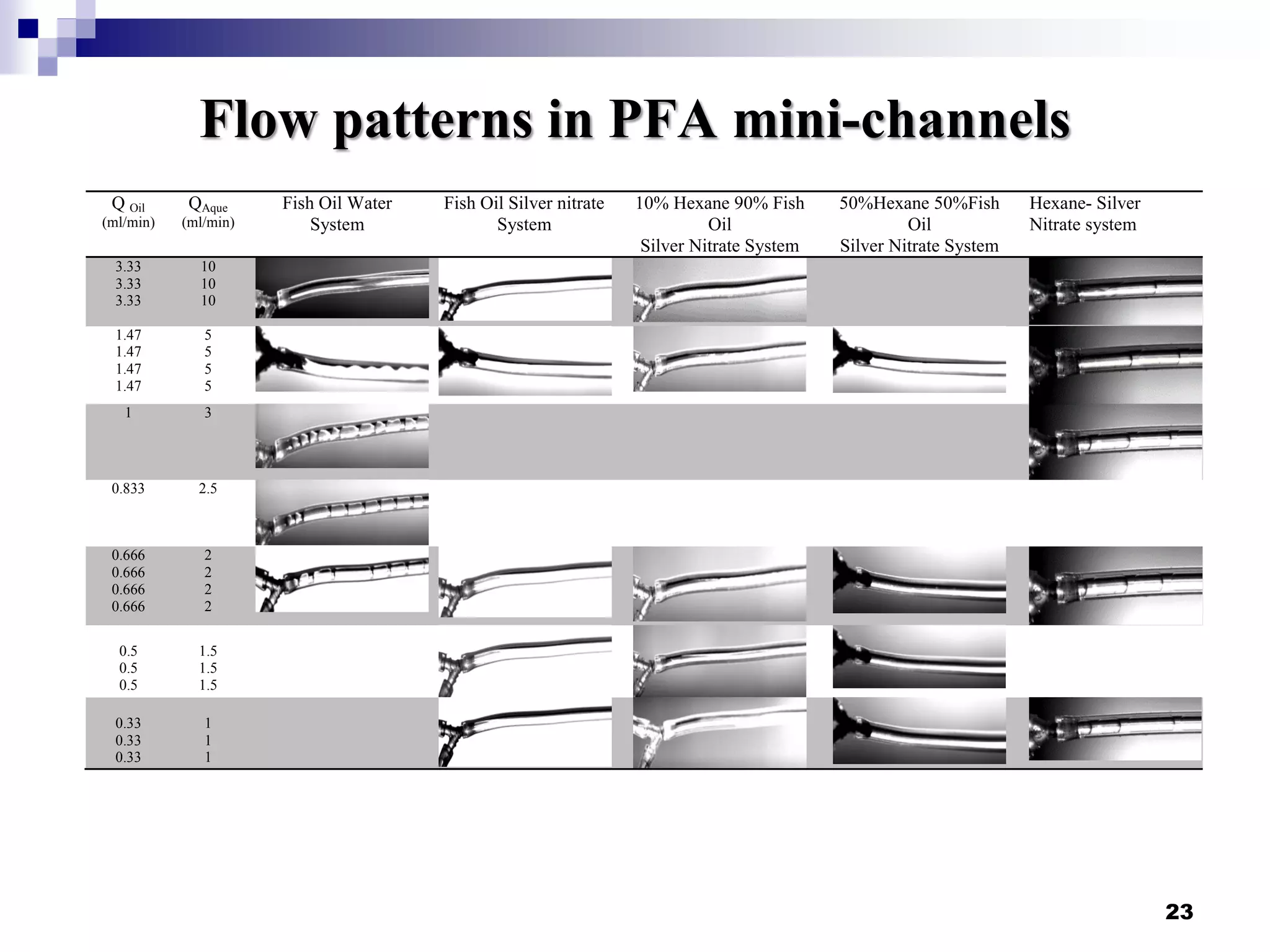
- Flow patterns in the mini-fluidic reactor deviated from the expected slug flow due to the properties of the actual fish oil and silver nitrate solutions used.
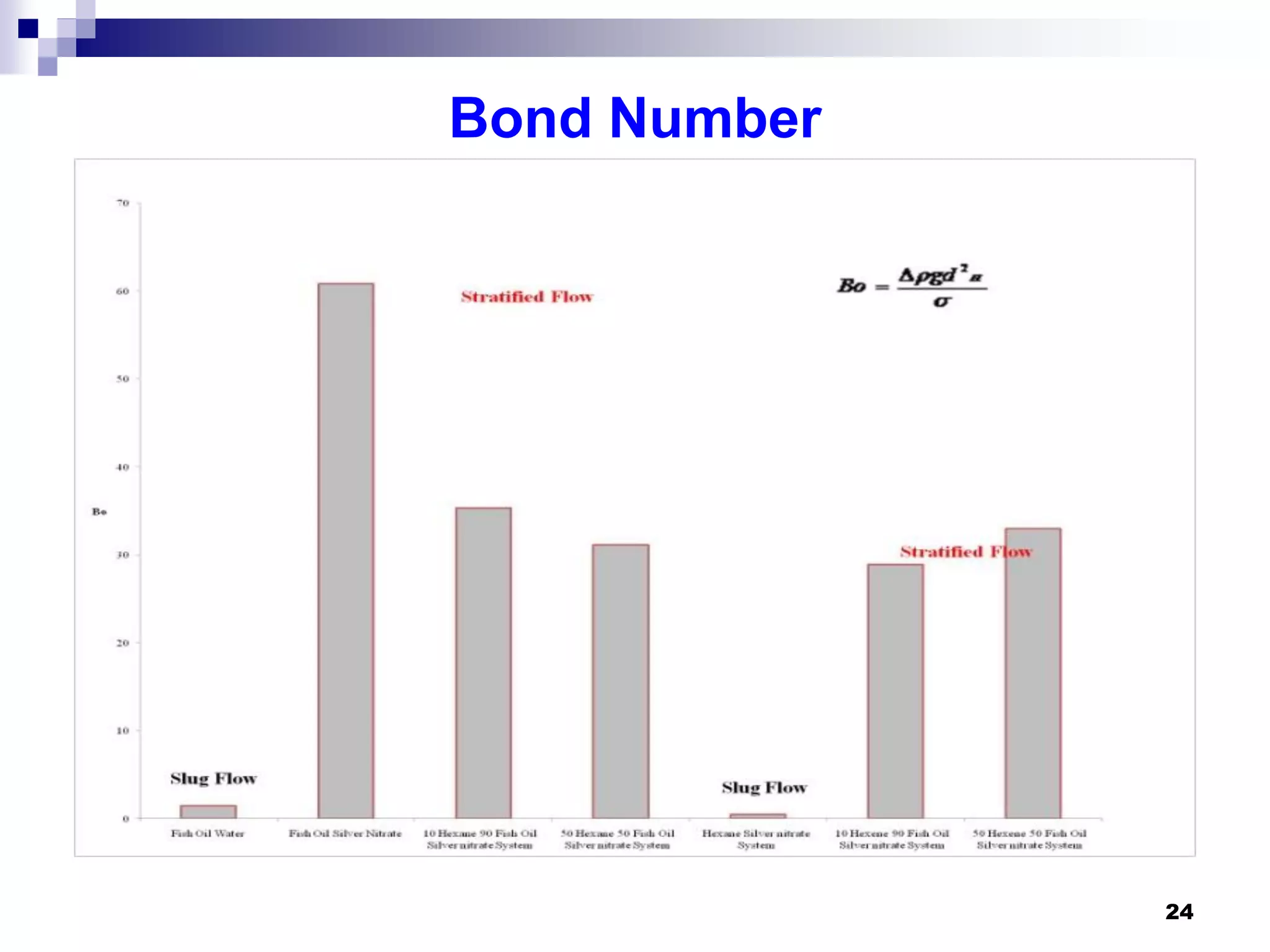
- Hydrodynamic studies showed stratified flow occurred rather than slug flow, indicating practical fish oil processing with silver nitrate may require handling stratified flows