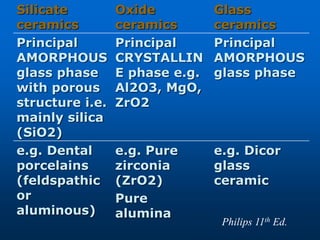
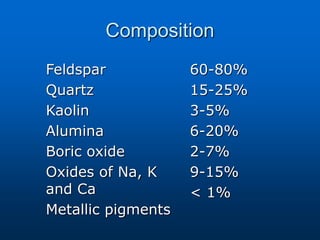
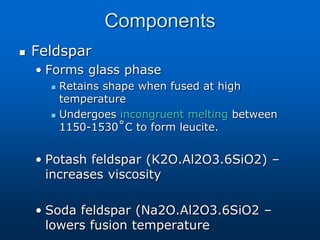
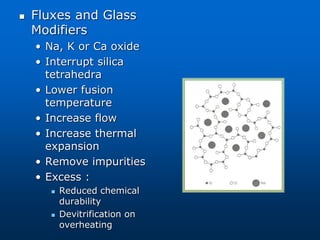
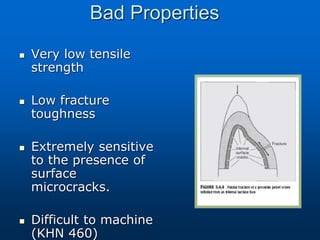
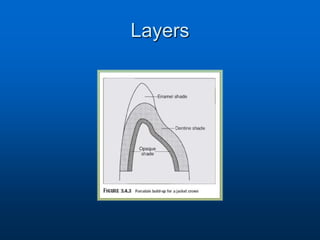
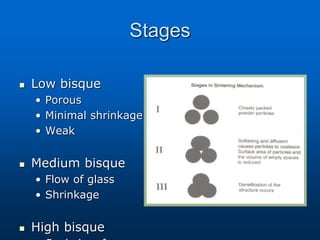
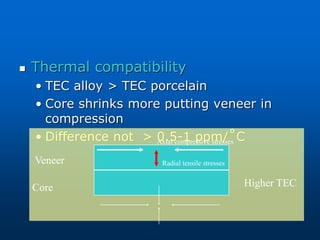
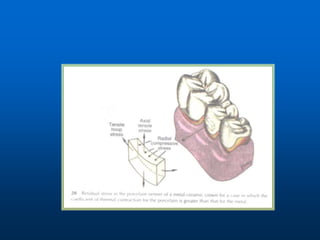
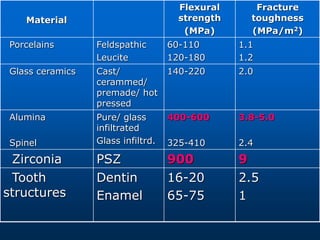
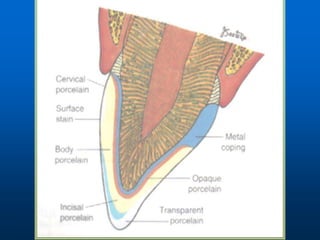
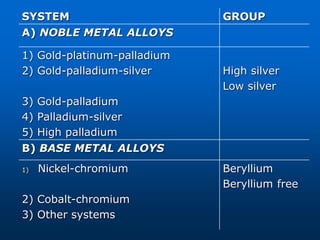
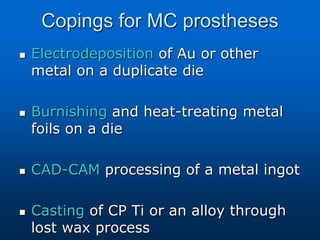
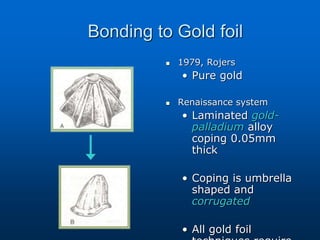
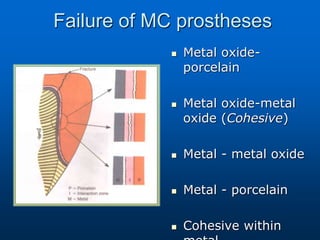
This document provides an overview of dental ceramics. It begins with definitions of ceramics and discusses the history and classification of dental ceramics. The main types described are silicate ceramics, oxide ceramics, and glass ceramics. The document then covers the composition, properties, processing methods, and strengthening techniques of various dental ceramic materials. It concludes with a section on metal ceramic systems, describing the advantages and types of alloys used.