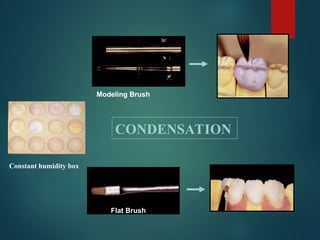
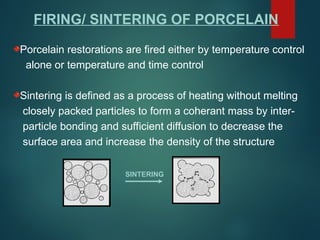
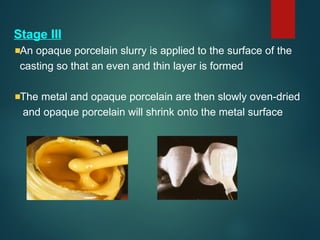
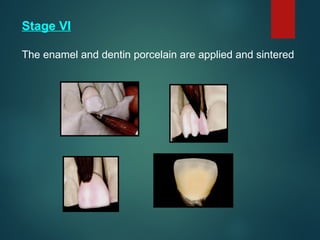
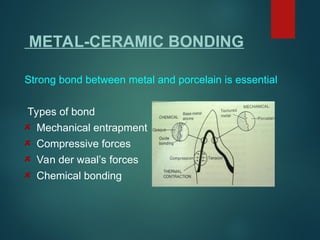
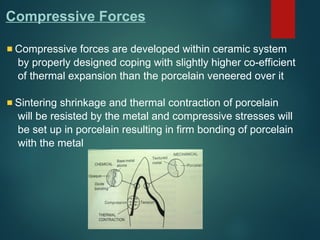
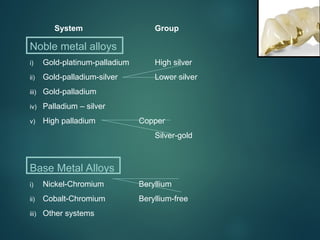
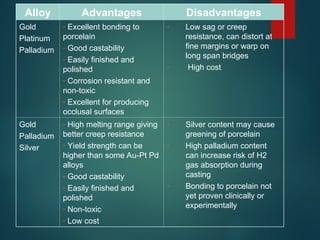
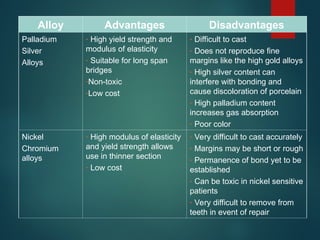
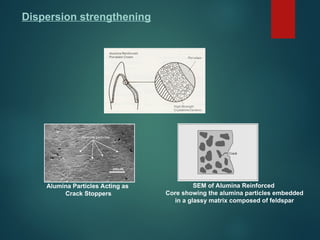
The document provides an extensive overview of dental ceramics, including their historical development, composition, classification, and fabrication processes. It discusses the types of porcelain and their properties, focusing on the requirements and benefits of metal-ceramic systems. Additionally, it covers the bonding mechanisms between metal and porcelain, types of metal-ceramic alloys, and the advantages and disadvantages associated with different materials used in dental restorations.