The document discusses a new technique for optimizing distillation blending and cutpoints in oil refineries to improve efficiency and control over product quality and yield. It involves manipulating true boiling point (TBP) curves by adjusting initial and final boiling points in order to optimize blending recipes and meet product specifications. The proposed method employs monotonic interpolation to replace traditional splines for enhanced accuracy in distillation temperature calculations, ultimately aiding in the production of ultra-low sulfur fuels.









![11
that the new flow for DC1 is less than the old flow and it is NF = 37.06 and OF = 39.24; consequently,
the flow for DC2 is 60.94, which is consistent with the total flow of 100.0. The new and optimized TBP
curve for DC1 given its front- and back-end shifts is now [(1.053%,312.8), (10.015%,432.9),
(31.188%,521.6), (52.361%,565.3), (73.534%,606.4), (94.707%,668.3), (98.995%,689.3)], where the
new yields are computed.
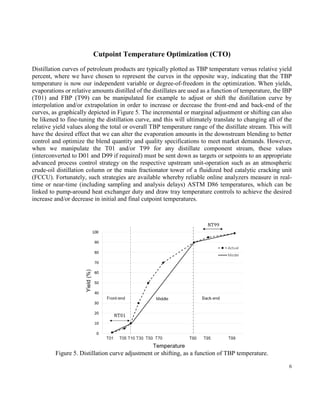
Figure 7. Example’s TBP distillation curves, including the final blend.
Distillation Blending and Cutpoint Temperature Optimization (DBCTO) in
Scheduling Operations
The cutpoint temperature optimization (CTO) is a conservative and adaptive TBP cutpoint method
to marginally/incrementally adjust/shift distillate yields when “non-sharp” (non-ideal, imperfect)
separation exists i.e., without an “infinite” number of stages and/or reflux. It uses ASTM D86
cutpoint temperatures or simulated distillation that are not as suitable as TBP temperatures given that
they over-predict the initial boiling-point (IBP) and under-predict the final boiling-point (FBP), but
these experimental methods are quicker to measure in the lab and field.
To apply the CTO method in the final fuel blending, we use the distillation curves of the distilled
streams from units such as CDU and FCCU to integrate their operation to variations in quantity and
quality of the produced intermediate fuels in order to fulfill final fuel demands in blendshops. Figure](https://image.slidesharecdn.com/kh1p8upfqdkpjubd4jwh-signature-25a4fbd7e158389994fb35b3168ee2c8e542e25112e4921d7f71db252b530173-poli-150923024525-lva1-app6891/85/Distillation-Blending-and-Cutpoint-Temperature-Optimization-in-Scheduling-Operations-11-320.jpg)

