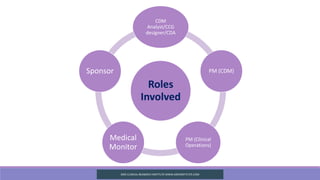
The document outlines CRF/e-CRF Completion Guidelines (CCG), which provide detailed instructions for accurately completing case report forms and facilitate electronic data capture. Its purpose is to improve data accuracy, offer traceability, and reduce downstream work related to data queries and audits. It also details the roles involved in creating CCGs, minimum standards for their development, and procedures for handling changes post-implementation.