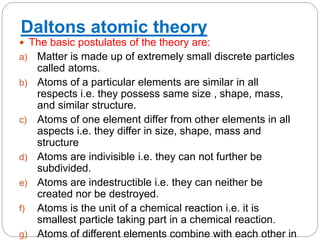
Dalton's atomic theory proposed that:
1) Matter is made of extremely small indivisible particles called atoms.
2) Atoms of a given element are identical in size, mass and properties.
3) Atoms of different elements vary in size, mass and properties.
The theory had limitations such as not explaining Gay-Lussac's law of combining volumes or the nature of atomic bonding.
Modern atomic theory has updated that:
1) Atoms can be subdivided into smaller particles like electrons and protons.
2) Atoms are destructible through nuclear processes converting mass to energy.
3) Atoms of a given element can differ in mass and properties as