Embed presentation
Downloaded 72 times

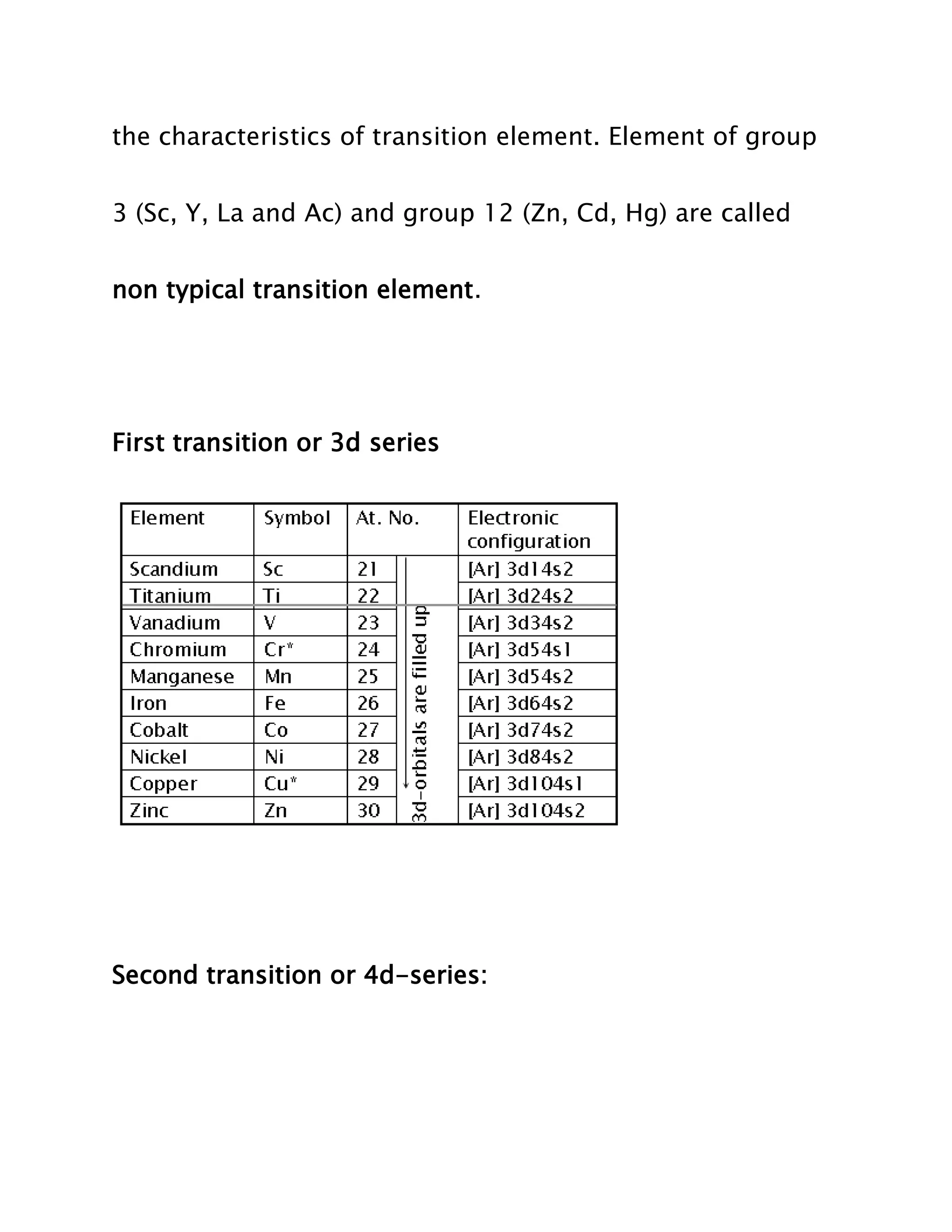
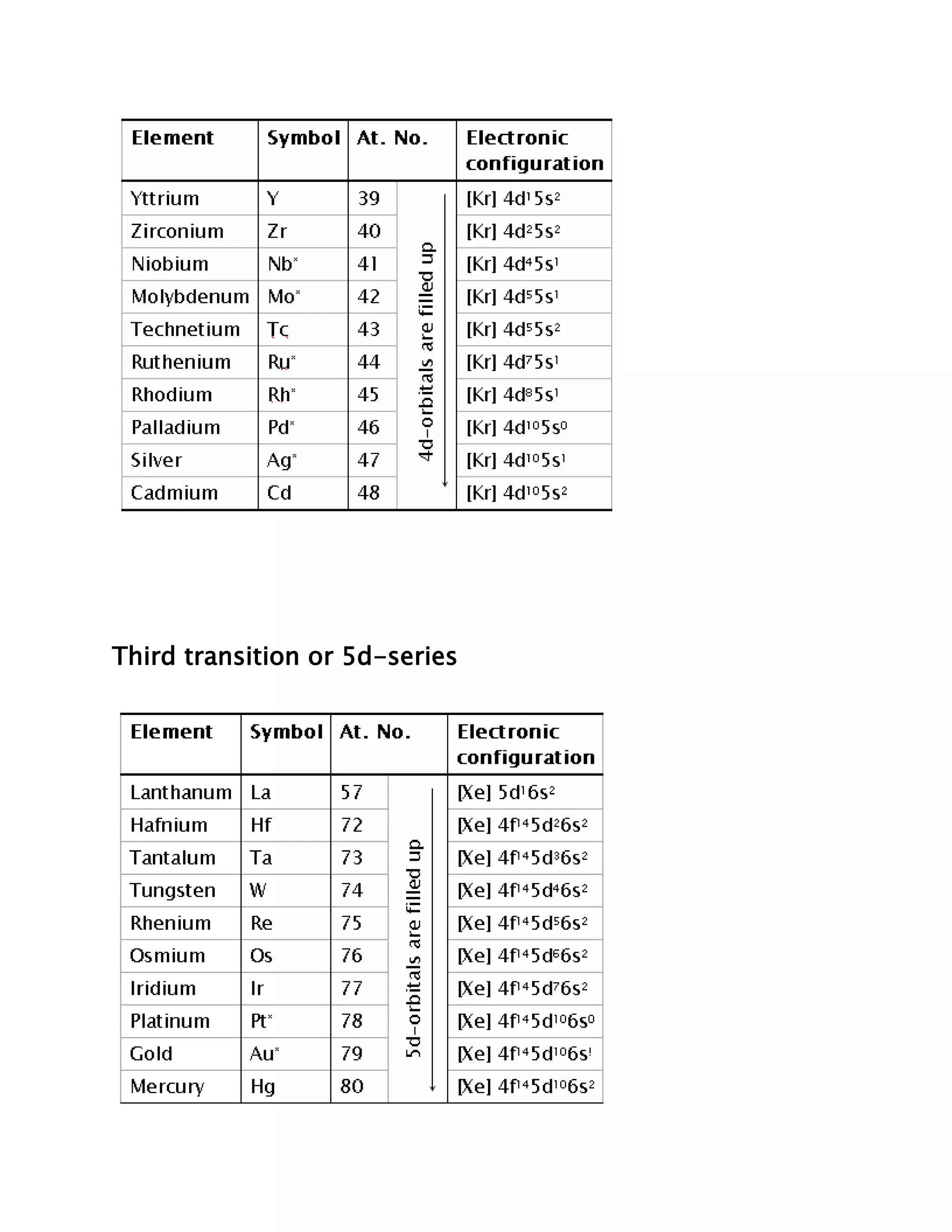
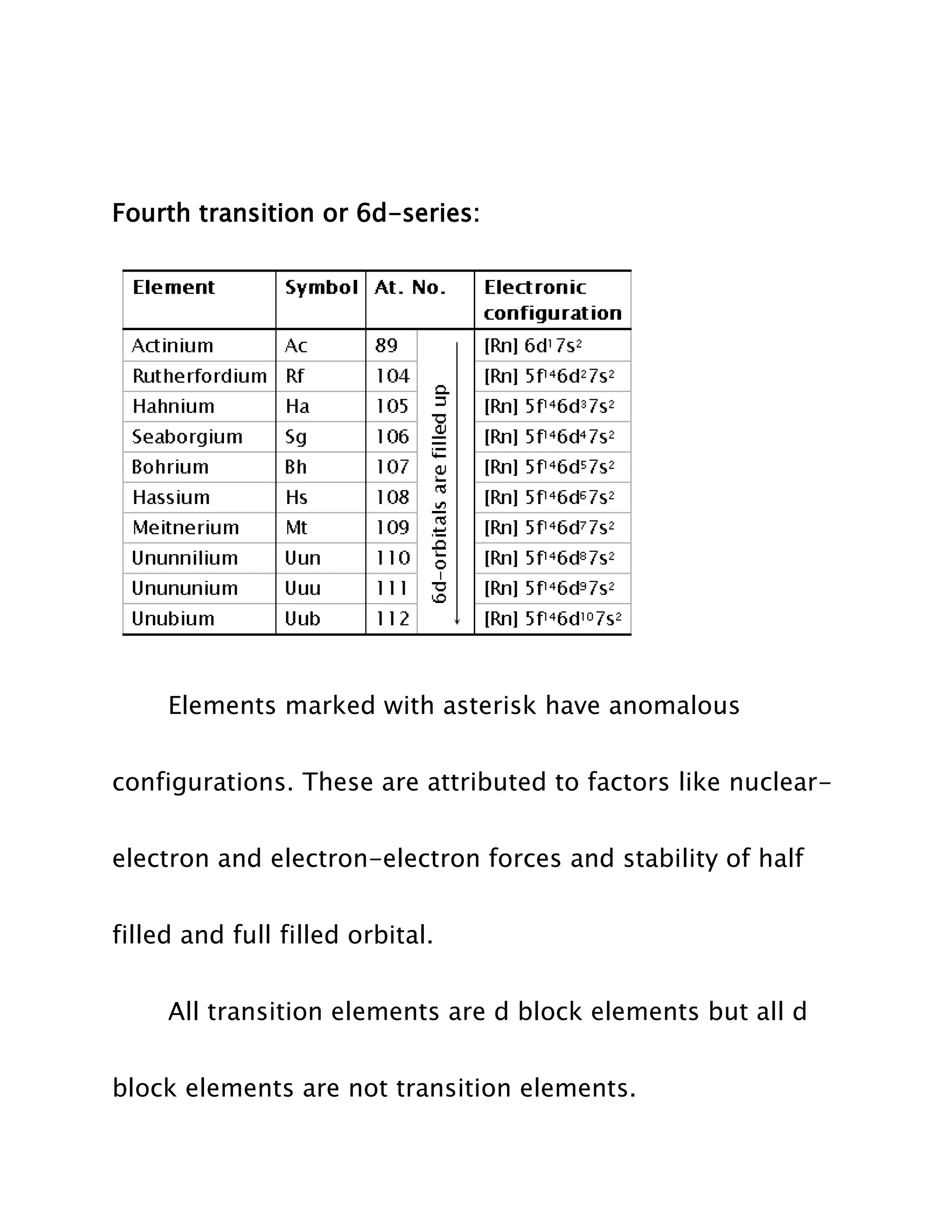


Transition elements are elements whose atoms have incomplete d sub-shells or whose ions have incompletely filled d orbitals. They lie between the highly electropositive s-block elements and highly electronegative p-block elements in the periodic table. While all transition elements are d-block elements, not all d-block elements are transition elements as some like zinc, cadmium, and mercury have completely filled d orbitals. Transition elements are divided into four series based on their electronic configurations.




