The document discusses the properties of d-block elements or transition elements. It describes their position in the periodic table, electronic configuration, and trends in various properties across the transition series. The key points are:
1) Transition elements have partially filled d orbitals and lie between the electropositive s-block and electronegative p-block elements in the periodic table.
2) Their electronic configurations follow the pattern [n-1]d1-10ns1-2 and there are three series of transition elements based on the d orbital - d-block, d-block and f-block.
3) Transition elements show variable oxidation states, high melting points, form colored compounds and alloys


![ELECTRONIC CONFIGURATION
[n-1]d1-10 ns1-2
AT.
NO.
21 22 23 24 25 26 27 28 29 30
ELE. Sc Ti V Cr Mn Fe Co Ni Cu Zn
E.C 3d1
4s2
3d2
4s2
3d3
4s2
3d5
4s1
3d5
4s2
3d6
4s2
3d7
4s2
3d8
4s2
3d10
4s1
3d10
4s2
First [3d] Transition element series[sc-zn]](https://image.slidesharecdn.com/xiidfblockelementsnotes-231015150513-ab497412/85/XII-d-f-block-elements-Notes-pptx-4-320.jpg)
![At. No. Element E.C
39 Y 4d1 5s2
40 Zr 4d2 5s2
41 Nb 4d4 5s1
42 Mo 4d5 5s1
43 Tc 4d6 5s1
44 Ru 4d7 5s1
45 Rh 4d8 5s1
46 Pd 4d10 5s0
47 Ag 4d10 5s1
48 Cd 4d10 5s2
At .No. ELEMENT
57 La
72 Hf
73 Ta
74 W
75 Re
76 Os
77 Ir
78 Pt
79 Au
80 Hg
E.C
5d1 6s2
5d2 6s2
5d3 6s2
5d4 6s2
5d5 6s2
5d6 6s2
5d7 6s2
5d9 6s1
5d10 6s1
5d10 6s2
Second [4d] transition series Third [5d] transition series](https://image.slidesharecdn.com/xiidfblockelementsnotes-231015150513-ab497412/85/XII-d-f-block-elements-Notes-pptx-5-320.jpg)
![The exceptional E.C of chromium
the 4s sub shell is completely filled
but 3d sub shell is neither
completely filled nor half filled .
• To attain a filled E.C Cu gains
one e- from the d-orbital and
attains the E.C of [Ar]d10
4s1.Thus both Cr and Cu have
exceptional E.C.](https://image.slidesharecdn.com/xiidfblockelementsnotes-231015150513-ab497412/85/XII-d-f-block-elements-Notes-pptx-6-320.jpg)
![TRANSITION ELEMENT
Elements which have partially filled [n-1]d
orbital’s in ground state or in excited state are
called transition element .
Zn ,Cd and Hg are not considered as transition
element because their ions, Zn2+,Cd2+ and Hg2+
have completely filled d-sub shell.](https://image.slidesharecdn.com/xiidfblockelementsnotes-231015150513-ab497412/85/XII-d-f-block-elements-Notes-pptx-7-320.jpg)

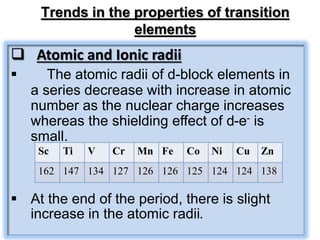

![Metallic character
• Except mercury which is a liquid ,all the
transition element have typical metallic
structure[hcp, ccp or bcc] They exhibits all the
characteristics of metals. For ex- They are
hard, lustrous, malleable and ductile etc.
Melting and Boiling point
• The transition metals have very high melting
and boiling point .The melting points of the
transition metals rise to a maximum and then
fall as the atomic number increases.](https://image.slidesharecdn.com/xiidfblockelementsnotes-231015150513-ab497412/85/XII-d-f-block-elements-Notes-pptx-11-320.jpg)


![• A very low value for Electrode [sc3+/Sc2+] reflects
the stability of Sc3+ ion which a noble gas
configuration
• For Sc Sc[21]= [Ar]3d1 4s2
• Sc[21]2+ = [Ar] 3d1 4s0
• Sc [21]3+= [Ar] 3d0 4s0
• The highest value for Zn is on account of very
high stability of Zn2+ ion with d10 configuration.](https://image.slidesharecdn.com/xiidfblockelementsnotes-231015150513-ab497412/85/XII-d-f-block-elements-Notes-pptx-14-320.jpg)
![• There is no regular trend in electrode [M2+/M]
values. This is because their ionization enthalpies
and sublimation enthalpies do not show any
regular trend.
• The general trend towards less negative electrode
values along the series is due to the general
increase in the sum of first and second ionization
enthalpies.
• Cu shows a unique behaviour in the series as it is
the only metal having positive value for
electrode.
Reason = As its sublimation and I.E does
compensate with hydration enthalpy.](https://image.slidesharecdn.com/xiidfblockelementsnotes-231015150513-ab497412/85/XII-d-f-block-elements-Notes-pptx-15-320.jpg)

