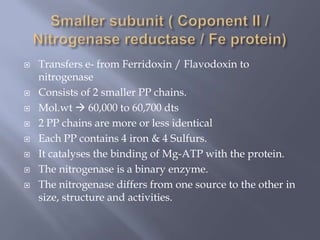
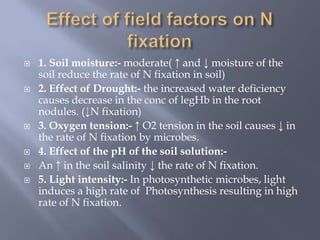
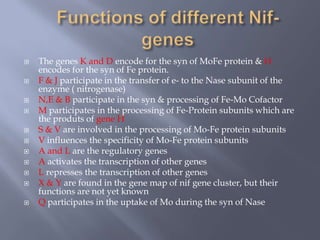
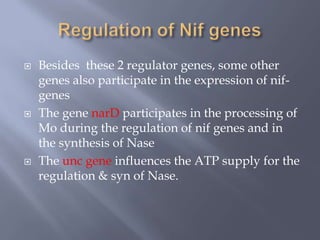
The document discusses nitrogen fixation and the nitrogen cycle. It notes that while nitrogen gas makes up 78% of the atmosphere, plants cannot use it directly and must obtain nitrogen from the soil in the forms of nitrates and ammonium salts. Nitrogen fixation is carried out through both biological and non-biological processes, with biological nitrogen fixation being the primary means of fixing atmospheric nitrogen in the soil through symbiotic bacteria like Rhizobium that form nodules on legume roots. The nitrogenase enzyme is responsible for this nitrogen fixation through an energy-intensive process requiring ATP.