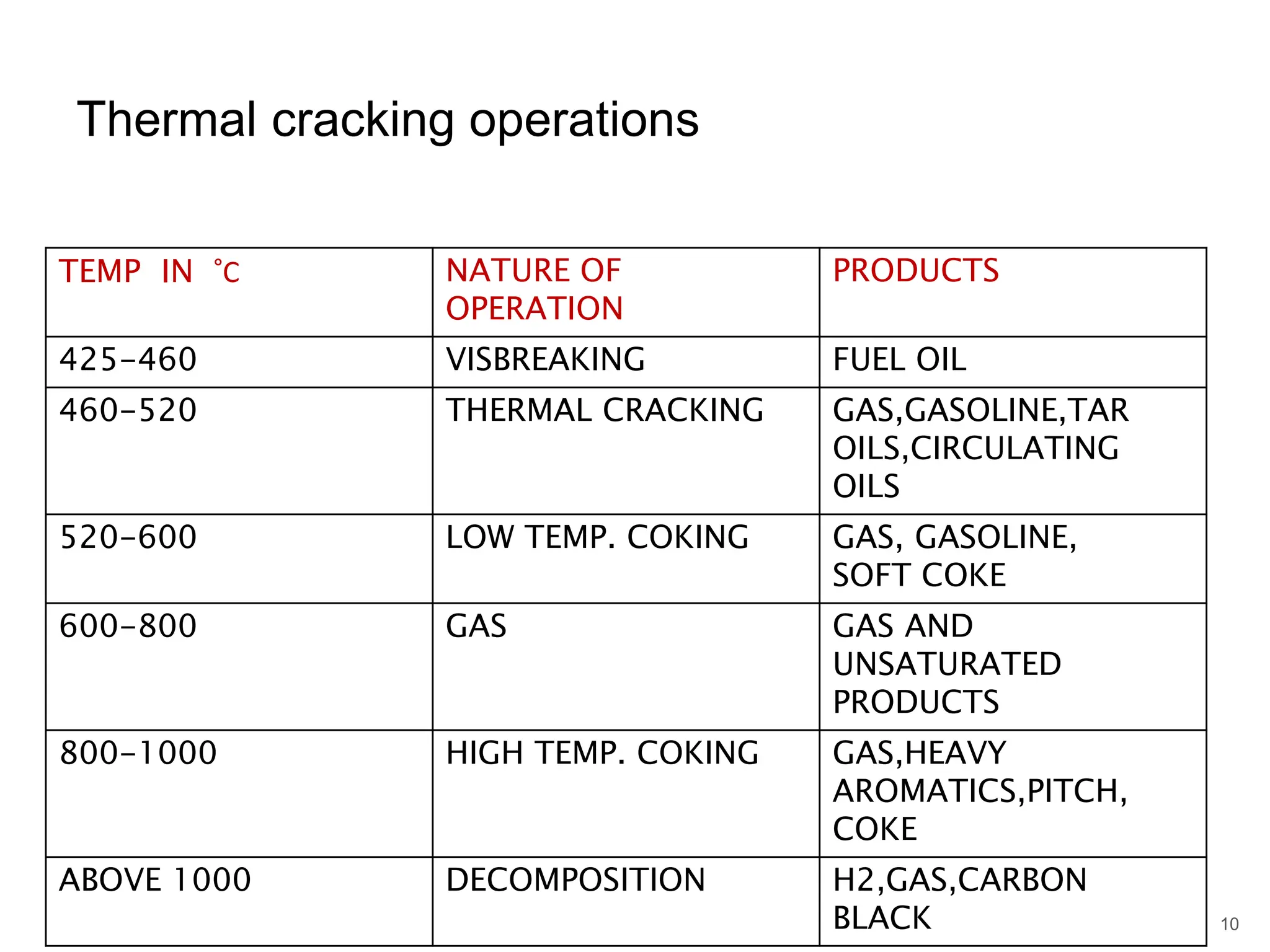
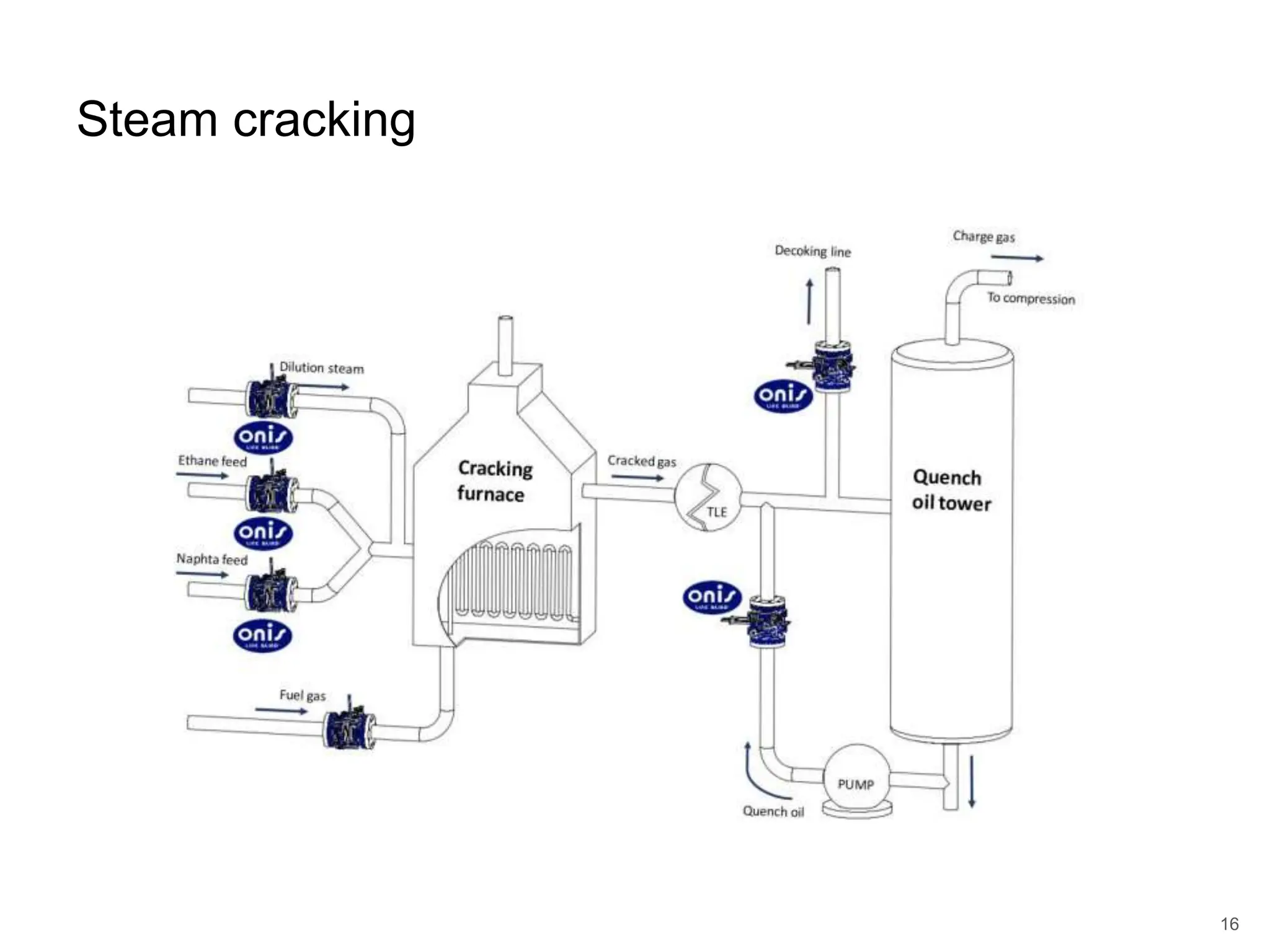
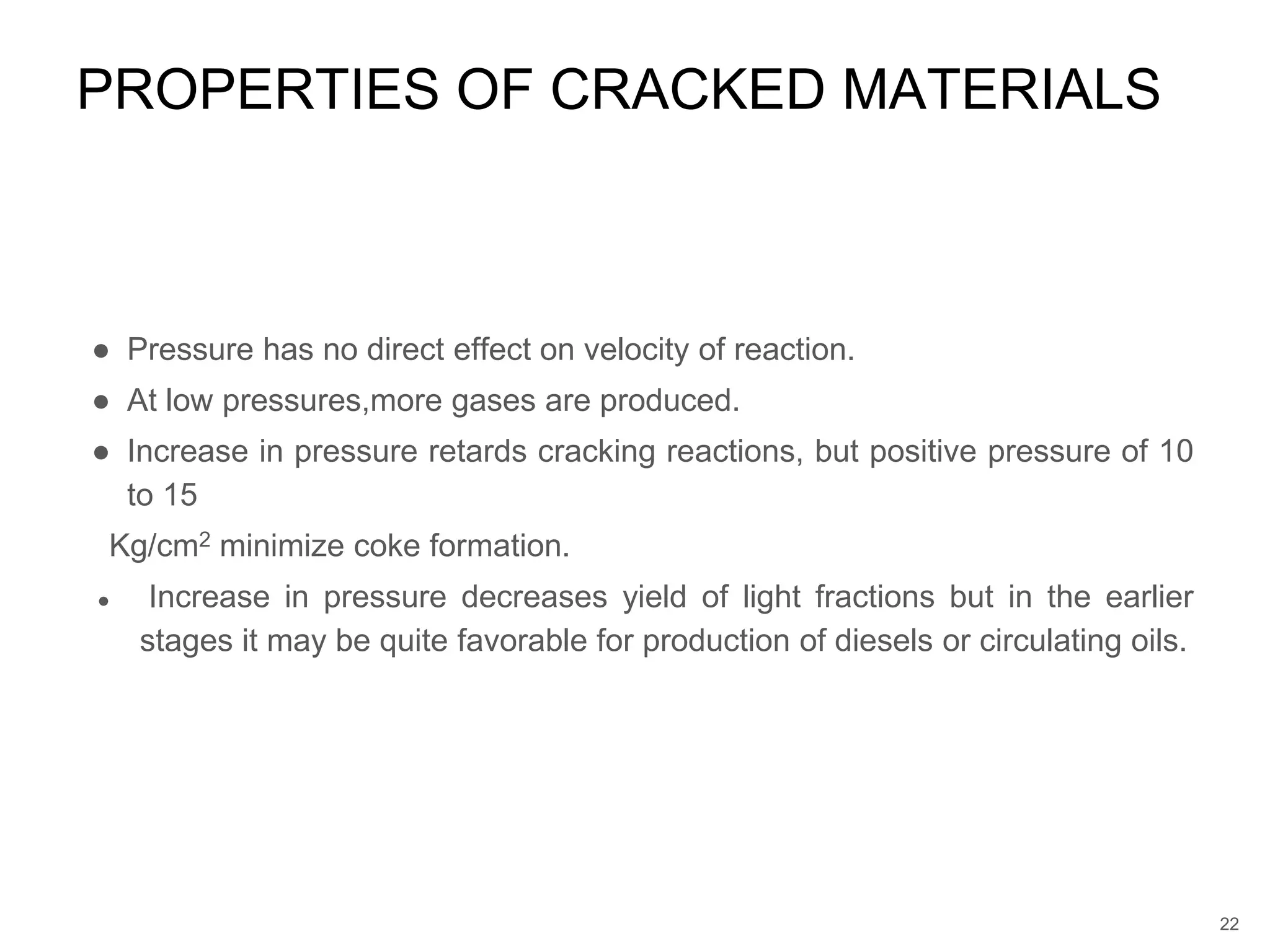
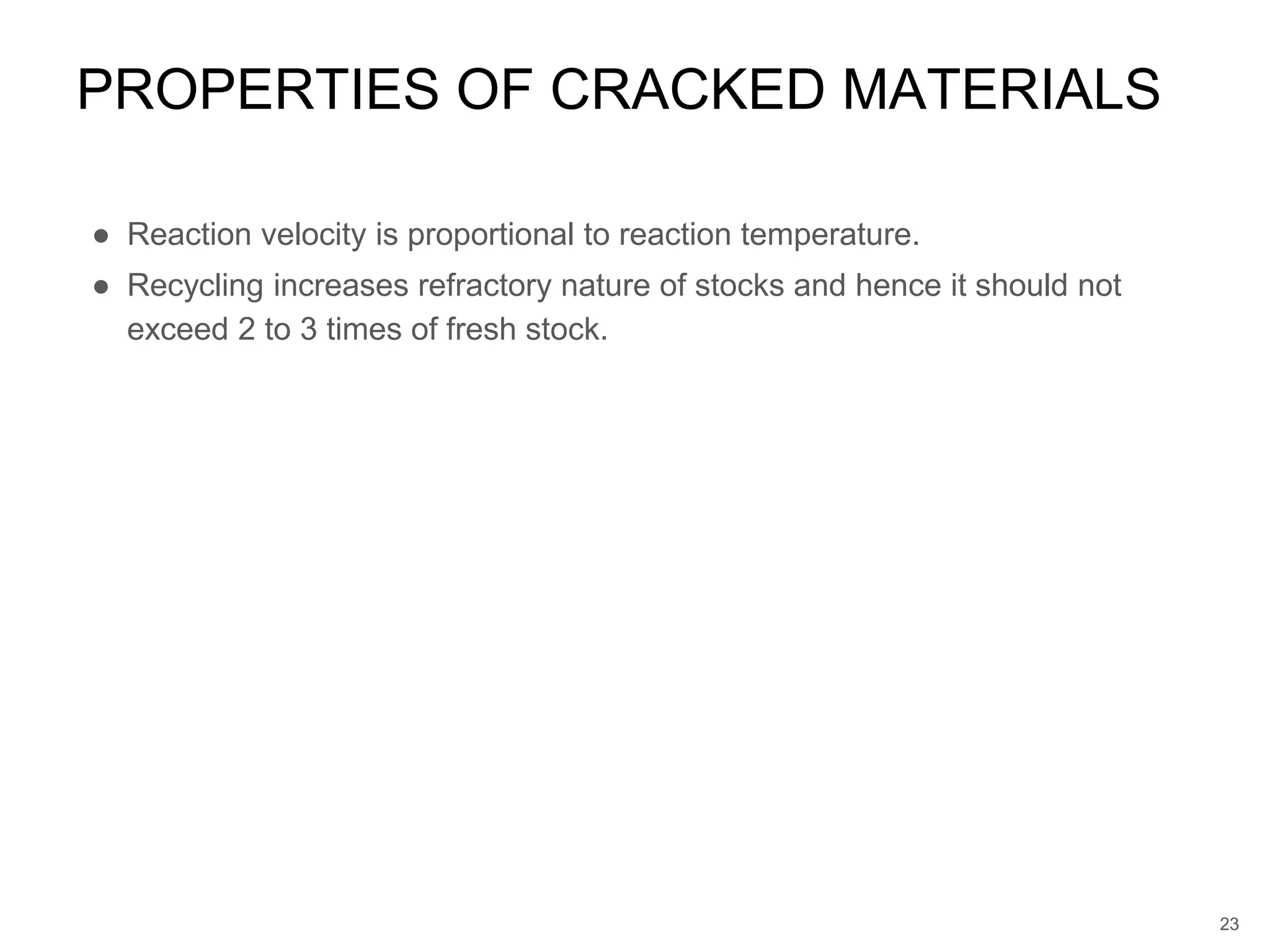
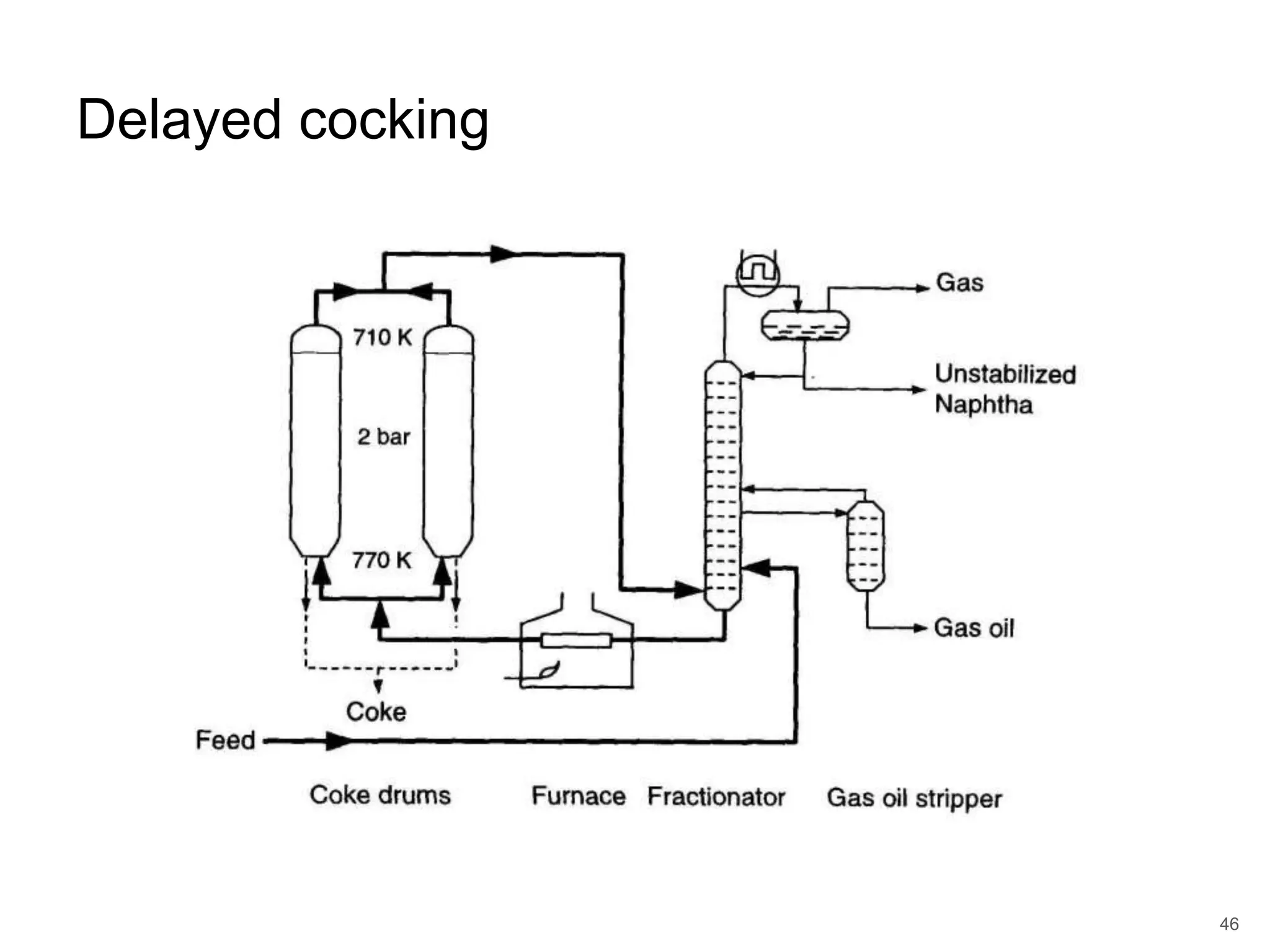
Cracking is a process that breaks down complex, heavy hydrocarbons like kerogen and heavy petroleum into lighter, more useful molecules like light hydrocarbons. There are two main types of cracking: thermal cracking uses high temperatures without a catalyst, while catalytic cracking uses lower temperatures with a catalyst. Cracking is necessary to produce important petrochemical feedstocks like ethylene and propylene that are in higher demand than heavier hydrocarbon fractions.