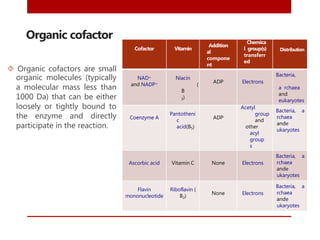
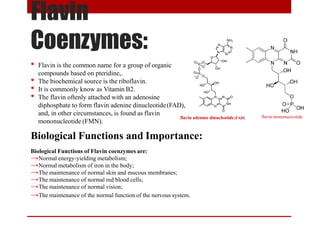
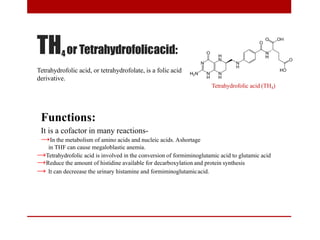
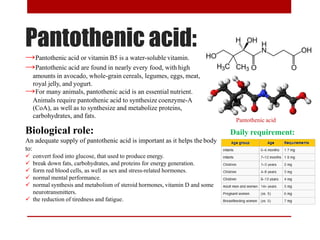
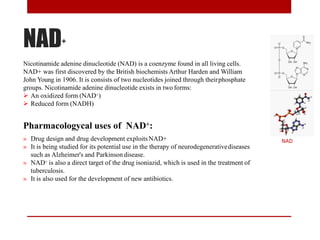
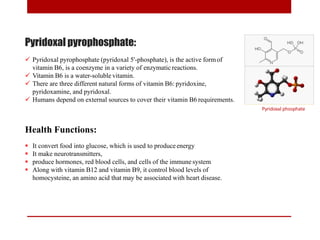
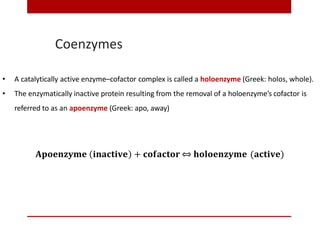
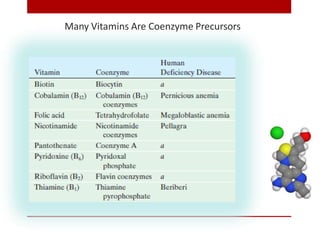
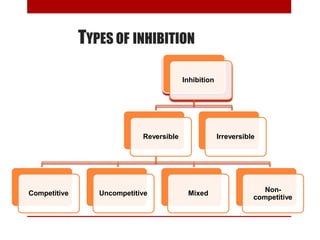
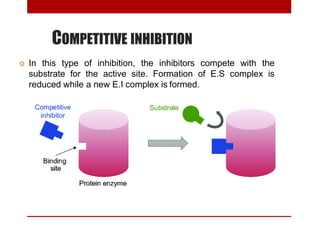
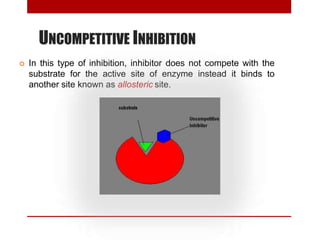
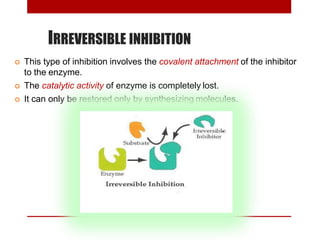
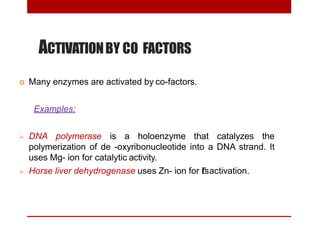
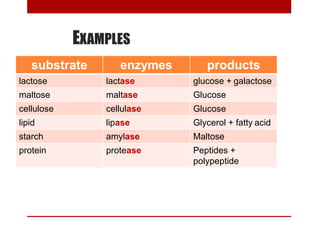
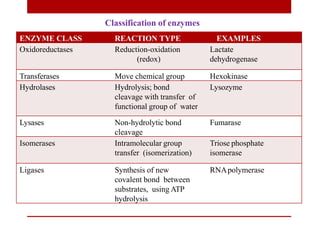
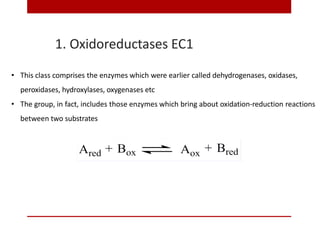
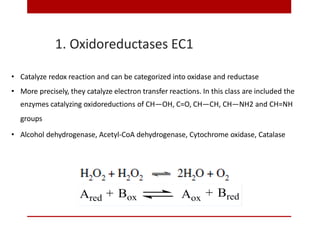
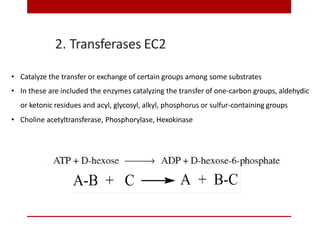
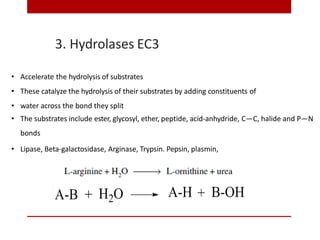
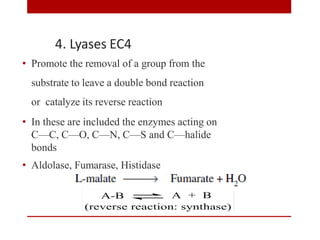
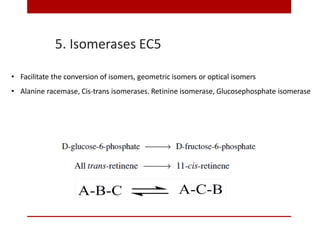
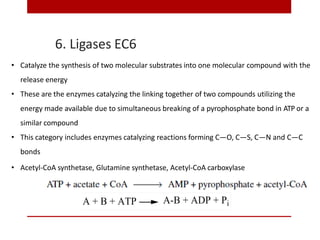
This document discusses enzymes and coenzymes. It begins by defining enzymes as protein catalysts that increase reaction rates without being used up in the process. It then discusses the classification, nomenclature, and properties of enzymes. The document also discusses coenzymes, which are organic molecules that bind to enzymes to form active holoenzymes. Several important coenzymes are described, including NAD+, FAD, thiamine pyrophosphate, biotin, and coenzyme A. The document emphasizes that coenzymes transfer chemical groups or electrons to carry out redox reactions and are regenerated at the end of the reaction cycle.




![Inorganic cofactor
Metal Ions
Ion Examplesof enzymes
containing this ion
Cupric Cytochrome oxidase
Ferrous or Ferric
Cy
Catalase
Heme)tochrome
(via
Nitrogen
ase
Hydroge
nase
Magnesium
Glucose 6-phosphatase
Hexokinase
DNA polymerase
A simple [Fe2S2] cluster containing two
iron atoms and two sulfur atoms,
coordinated by four protein cysteine
residues.](https://image.slidesharecdn.com/xiiilessonenzyme-201206103413/85/Enzyme-22-320.jpg)