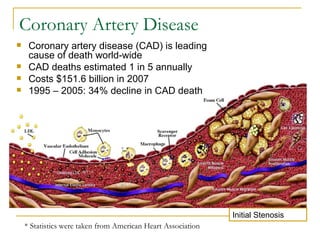
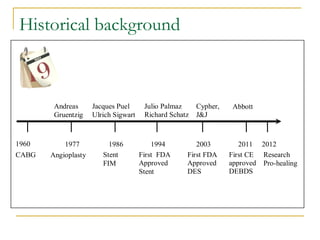
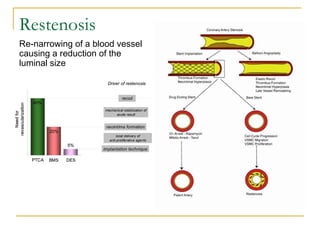
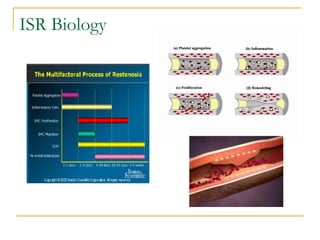
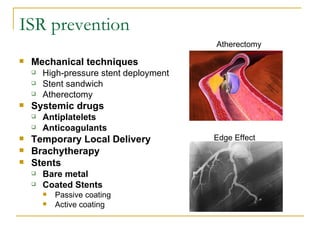
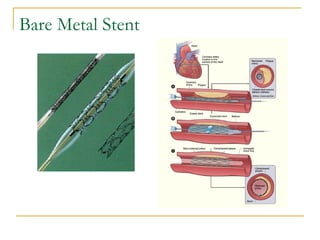
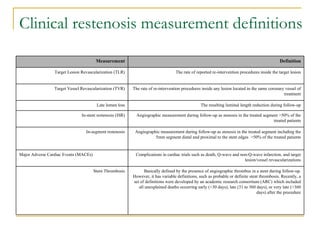
Stent design aspects and coronary artery disease were discussed. Coronary artery disease is a leading cause of death worldwide. Historical developments in treatments including balloon angioplasty, bare metal stents, and drug-eluting stents were covered. Mechanisms of in-stent restenosis and approaches to prevent it such as mechanical techniques, drug coatings, and biodegradable stents were described. Clinical measures for evaluating restenosis outcomes were defined.